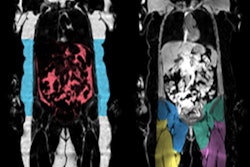
Swedish image analysis software developer AMRA Medical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Muscle Assessment Score (MAsS) Scan software.
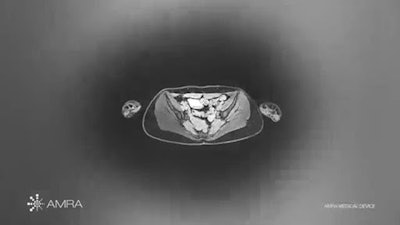 AMRA's MAsS Scan software provides muscle assessment on MRI exams. Image courtesy of AMRA Medical.
AMRA's MAsS Scan software provides muscle assessment on MRI exams. Image courtesy of AMRA Medical.MAsS Scan analyzes muscle and fat biomarkers on a neck-to-knee MRI scan to produce a patient-specific report that contains the muscle assessment score, anatomic and color-coded images, and precise body composition measurements with contextual insights, according to the vendor. AMRA also recently received a license to distribute MAsS Scan in Canada.