The combination of gadolinium and ultrasmall superparamagnetic iron oxide (USPIO) particles in patients with multiple sclerosis (MS) reveals 51% more active lesions in MRI scans than gadolinium alone, French researchers report in the July issue of Radiology.
A research team led by Dr. Thomas Tourdias, PhD, of the Université Bordeaux Segalen, found that lesions enhanced by both contrast agents were larger and exhibited a more aggressive evolution than lesions that were visible with only one contrast agent, even on follow-up images after six months (Radiology, July 2012, Vol. 264:1, pp. 225-232).
MRI with a gadolinium-based contrast agent has been the modality of choice in MS patients to determine a diagnosis, assess disease activity, select therapeutic treatment, and evaluate treatment efficacy. Traditionally, USPIO nanoparticles have been used to track macrophages in the central nervous system; previous research has proved their feasibility in patients with multiple sclerosis, according to the authors. However, use of USPIO in the progressive forms of MS has not been investigated.
As a result, Tourdias and colleagues recruited 24 patients at least 18 years of age with relapsing and progressive forms of MS who were undergoing treatment at the time. Patients were excluded if they had been treated with steroids during the previous two months, had contraindications to MRI, allergies to dextrans or drugs containing iron salts, were pregnant or breast-feeding, or had received iron oxide nanoparticles within seven days before USPIO-enhanced imaging.
The subjects underwent 1.5-tesla MRI scans (Philips Healthcare, Siemens Healthcare) at baseline and at a six-month follow-up date. Each patient received an MRI scan with gadolinium (Dotarem, Guerbet) and an MRI scan one day later with USPIO.
The first MRI scan included axial T1-weighted spin-echo images. USPIO-enhanced MRI included axial T1-weighted, T2-weighted, and T2*-weighted sequences.
Immediately after the first MRI exam, USPIO (Sinerem, Guerbet, also known as Combidex, AMAG Pharmaceuticals) was injected over approximately 30 minutes (2.6 mg of iron per kg of body weight diluted in 100 mL of saline) and MR imaging was performed 24 to 48 hours later.
For both baseline and six-month follow-up MRI scans, 30 images were obtained 24 hours after USPIO administration and 14 images were acquired 24 to 48 hours after USPIO administration.
Two readers independently evaluated images from 24 patients at baseline, with data available from six-month follow-up scans for 20 patients (83%). Three patients did not undergo follow-up after experiencing USPIO-related side effects at baseline, while one patient was lost to follow-up.
Active lesions
Among the 24 patients evaluated at baseline, 1,287 lesions were counted with T2-weighted MR imaging, with 56 active lesions discovered with gadolinium and/or USPIO. Of those 56 lesions, 37 (66%) in 10 patients were enhanced with gadolinium (nine lesions with gadolinium alone and 28 lesions with USPIO and gadolinium).
Use of USPIO allowed the researchers to identify 19 additional lesions (34% of all enhanced lesions, or 51% more lesions than with gadolinium alone), which upgraded the diagnosis on two patients to having active MS.
 |
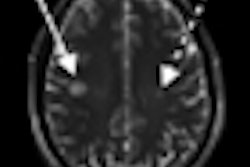
| MR images were obtained at baseline in a 49-year-old woman with progressive multiple sclerosis prior to treatment. Right periventricular lesion seen on axial T2-weighted image did not enhance on T1-weighted image obtained a few minutes after gadolinium injection, whereas images obtained 24 to 48 hours after USPIO injection show mild enhancement at T1-weighted imaging with no signal loss at T2-weighted imaging (arrows). All images courtesy of Radiology. |
All 47 lesions enhanced by USPIO (28 lesions with USPIO and gadolinium; 19 with USPIO alone) exhibited high signal intensity on T1-weighted MR images. Twenty-five (53%) of the 47 lesions had low signal intensity on T2-weighted images and even lower signal intensity on T2*-weighted images at the same location.
The researchers also determined that a significantly greater number of enhanced lesions were found in the relapsing patient group (46 of 429 lesions, 11%) than in the progressive patient group (10 of 858 lesions, 1%).
The results were similar for USPIO and gadolinium, with the methods helping to identify five additional lesions in the progressive group and 14 additional lesions in the relapsing group.
Of the 56 lesions that were enhanced by the contrast agents at baseline, 53 (95%) could be analyzed at the six-month mark. Seven (26%) of the 27 lesions that enhanced with both USPIO and gadolinium at baseline showed enhancement with both agents at six-month follow-up, compared with only one (4%) of 26 lesions that enhanced with only gadolinium or USPIO.
Safety
Adverse events were reported in nine (38%) of the 24 patients at baseline and in four (20%) of the 20 patients at six-month follow-up.
"The most-frequent adverse events were pain in the extremities, headache, dyspnea, rash, and urticaria," the authors wrote. All of these symptoms disappeared with no lingering effects.
Tourdias and colleagues cited several limitations of their study, such as the small number of patients in the sample. "Nevertheless," they added, "it represents the largest study of USPIO administration in patients with [multiple sclerosis] to date. In addition, the short follow-up duration does not allow firm conclusions to be made regarding the predictive value of clinical progression."
Even with the limitations, the study supports the "potential advantages of using both a gadolinium-based contrast agent and USPIO in patients with [multiple sclerosis] to increase the sensitivity of MR imaging in the detection of disease activity, even in progressive forms, and suggests that lesions that enhance with both contrast agents represent a subgroup with more severe features," the authors concluded.