Philips Healthcare has garnered U.S. Food and Drug Administration (FDA) 510(k) clearance for its Ingenia Elition 3-tesla MRI scanner.
First announced at ECR 2018 in March, the scanner features the company's Compressed SENSE MRI and 3D APT protocols, which have also received FDA 510(k) clearance. The Compressed SENSE MRI acceleration technique is a parallel imaging mode that increases the speed at which data can be transferred from scanners, while 3D APT is a contrast-free brain MRI application that can assist radiologists with tasks such as differentiating between low- and high-grade gliomas, according to the vendor.
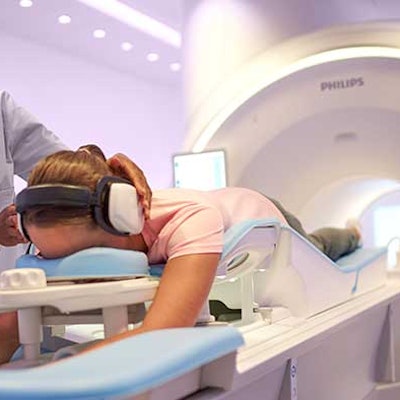
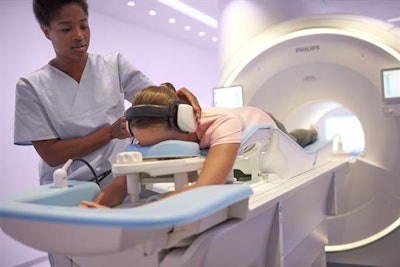 The Ingenia Elition 3-telsa MRI system. Image courtesy of Philips Healthcare.
The Ingenia Elition 3-telsa MRI system. Image courtesy of Philips Healthcare.In other features, the firm's VitalEye application enables an "intelligent" respiratory signal that allows routine exams to be set up in less than a minute, Philips said. Elition also utilizes the company's SmartExam analytics software for automatic planning, scanning, and processing of exams.
The scanner can also be paired with the vendor's Ambient Experience in-bore Connect technology to provide an immersive audiovisual experience for patients, according to the vendor. Philips recently delivered the first commercial Elition system to Hennepin Healthcare in Minneapolis. Elition is also available in Europe.