Lantheus Medical Imaging has received approval from the U.S. Food and Drug Administration (FDA) for Pylarify (F-18 DCFPyL), a PET radiopharmaceutical designed to target prostate-specific membrane antigen (PSMA).
Pylarify is labeled with F-18 and homes in on PSMA, a protein that is overexpressed on the surface of more than 90% of both primary and metastatic prostate cancer cells, according to the company. PET imaging can detect accumulations of the agent at sites of prostate cancer, making Pylarify useful for detecting metastasis from primary cancer or recurrence of disease in men who have already been treated.
Lantheus believes that clinicians will be able to use Pylarify to detect emerging prostate cancer faster than other imaging tools and help them develop treatment plans. The company noted that 50% of men who are treated for prostate cancer experience recurrence within 10 years of therapy.
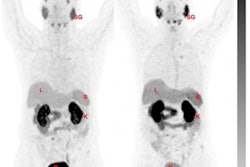
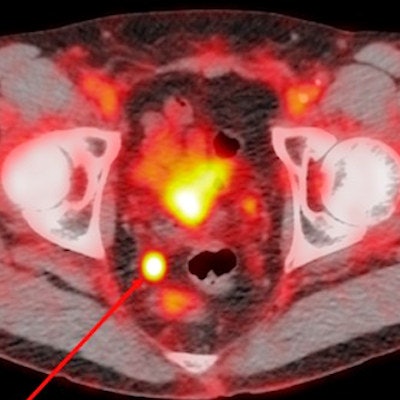
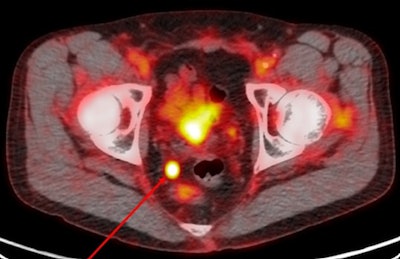 Image acquired with Pylarify demonstrates radiotracer uptake in right perirectal lymph node. Image courtesy of Lantheus.
Image acquired with Pylarify demonstrates radiotracer uptake in right perirectal lymph node. Image courtesy of Lantheus.The company further noted that while recurrence of prostate cancer can be detected with blood tests for prostate-specific antigen (PSA) blood serum levels, it can be difficult for conventional imaging to precisely locate the origin of the cancer, especially in men with low PSA levels.
PSMA is expressed in 90% of prostate cancers, and Pylarify binds to targets to enable PET detection. The F-18 tracer used with the radiopharmaceutical is produced in cyclotrons and has a 110-minute half-life, which Lantheus said allows for wider geographic distribution.
In support of Pylarify's regulatory submission, Lantheus supplied data from two clinical studies, OSPREY and CONDOR. The OSPREY study found higher specificity and positive predictive value in Pylarify scans of men prior to initial therapy. The CONDOR study focused on men with biochemical recurrent prostate cancer in which conventional imaging was unclear and found that Pylarify had high rates of localization and detection, even in men with PSA values at a median of 0.8 ng/mL.
Lantheus said that Pylarify will be available immediately in the mid-Atlantic and southern U.S., with availability expanding over the next six months. Availability across the entire U.S. is expected by the end of the year.