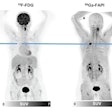
Black men have less access to PET imaging for recurrent prostate cancer with a radiotracer that appears to be more effective than the more commonly used F-18 fluciclovine, according to a study published September 25 in the Journal of Nuclear Medicine.
Since gallium-68 (Ga-68) prostate-specific membrane antigen (PSMA)-11 appears to perform better than F-18 fluciclovine, patient access is key, wrote a team led by Dr. Matthew Bucknor of the University of California, San Francisco (UCSF). And although Black men have higher rates of incidence and mortality due to prostate cancer (due to a variety of factors, from socioeconomics and discrimination to access to healthcare and delayed disease diagnosis), they may not have equal access to cutting-edge diagnosis and treatment.
"Recent studies have shown that Ga-68 PSMA-11 offers significantly improved detection rates compared to F-18 fluciclovine," the team wrote. "However, Ga-68 PSMA-11 is not yet [U.S. Food and Drug Administration] approved and has only been accessible in the U.S. through clinical trials ... The purpose of this study was to ... identify potential disparities in access to state-of-the-art care in intermediary steps of health delivery, which may contribute to disparities in prostate cancer health outcomes."
The U.S. Food and Drug Administration (FDA) cleared F-18 fluciclovine (sold commercially as Axumin) for prostate cancer imaging in 2016, and the tracer is reimbursed by both public and private healthcare insurers. However, Ga-68 PSMA-11 is still considered investigational and can be administered only to patients who are participating in clinical trials, such as those that were underway at UCSF.
To investigate the accessibility of Ga-68 PSMA-11 among men with recurrent prostate cancer, Bucknor and colleagues conducted a study that included 1,756 patients who were enrolled in clinical trials that were underway at UCSF. Of these, 1,502 underwent PET imaging with Ga-68 PSMA-11 and 254 who underwent PET imaging with F-18 fluciclovine between October 2015 and January 2020. The team tracked patients' race and ethnicity, primary language, body mass index, insurance type, and home addresses; the addresses were used to identify patients' socioeconomic status with Census Block Group, a geographical unit used by the U.S. Census Bureau.
The group found that Black patients were almost four times more likely to undergo F-18 fluciclovine PET imaging versus Ga-68 PSMA-11 when compared with white patients (OR, 3.88), despite the fact that the researchers found no statistically significant demographic factors between the two groups. The team also noted that even though participation in an ongoing clinical study was required for access to Ga-68 PSMA-11, six times as many patients were imaged with the tracer, which highlights "potential disparities in access to imaging research trials for Black patients," it wrote.
The study findings underline an urgent need to address healthcare disparities -- especially since the reasons for the differences are unclear, although they likely include unconscious racial bias, according to Bucknor and colleagues.
"The current study demonstrates the essential need for more studies of this kind in radiology as a critical precondition for developing policies and procedures that can identify and eliminate structural barriers to equitable care delivery," they concluded.