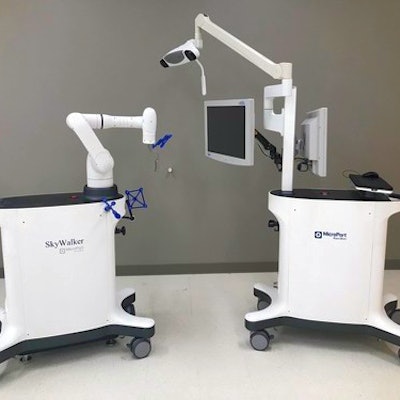
Robotic surgery technology developer MicroPort Navibot has garnered U.S. Food and Drug Administration (FDA) 510(k) clearance for SkyWalker, its first robot-assisted platform targeted at orthopedic applications.
SkyWalker's planning system first assists surgeons in formulating personalized patient implants based on preoperative CT images as well as specific implant data, according to the vendor. Then during surgery, SkyWalker facilitates implant positioning to achieve the desired patient-specific kinematics, MicroPort said. Next, surgeons use the system's mechanical arm to proceed to resection.
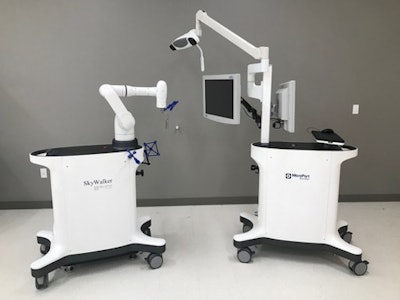 MicroPort Navibot's SkyWalker robot-assisted surgery platform for orthopedic applications.
MicroPort Navibot's SkyWalker robot-assisted surgery platform for orthopedic applications.Initially, SkyWalker will feature a robotically assisted total knee replacement procedure compatible with MicroPort's Evolution medial-pivot knee system, according to MicroPort. The company is currently developing other orthopedic applications in partnership with sister company MicroPort Orthopedics.