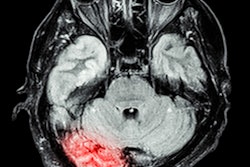
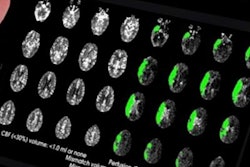
Cerebrovascular imaging software developer iSchemaView has received an additional clearance from the U.S. Food and Drug Administration (FDA) for its Rapid neuroimaging platform, enabling its use in identifying stroke patients who are likely to benefit from a clot removal procedure.
Under the new clearance, the company's Rapid CT-Perfusion and MR-Perfusion software can now also be used by physicians to help select acute stroke patients with known occlusion of the internal carotid artery or proximal middle cerebral artery for endovascular thrombectomy, according to the vendor.