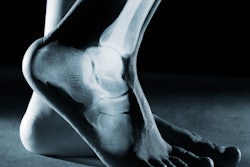
CurveBeam has received U.S. Food and Drug Administration (FDA) 510(k) clearance for InReach, a conebeam CT imaging system primarily designed for the hand, wrist, and elbow, and lower extremities in nonweight-bearing positions.
InReach is a compact CT scanner that provides high-contrast 3D images of bony anatomy, is self-shielded, and has standard power requirements. Hands, wrists, or elbows can be positioned in a height-adjustable bore while patients are standing or sitting. The unit can also accommodate nonweight-bearing lower-limb imaging. Scan times are less than 30 seconds, according to the firm.
The InReach device is supplemented by CubeVue, CurveBeam's custom visualization software that provides multiplanar slices and 3D renderings of anatomy. CubeVue's Insta-X feature provides digitally reconstructed x-rays.