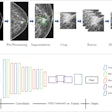
Advanced visualization firm TeraRecon said that the U.S. Food and Drug Administration (FDA) has determined that its Northstar AI Results Explorer application, which provides artificial intelligence (AI)-assisted workflows, is exempt from class II premarket notification requirements and is therefore considered a class I medical device.
Designed to work with the company's EnvoyAI interoperability platform, Northstar AI Results Explorer enables radiologists to view and interact with the results of AI algorithms directly within their workflow, according to the vendor. EnvoyAI currently provides access to more than 80 AI algorithms, including 20 that have received regulatory clearance in various global territories, the company said.