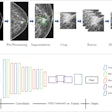
The U.S. Food and Drug Administration (FDA) is working on a new regulatory framework aimed at encouraging the use of artificial intelligence (AI) in healthcare, according to a report from the Hill.
In prepared comments on April 26 at the Health Datapalooza conference in Washington, DC, FDA Commissioner Dr. Scott Gottlieb said that the updated framework would allow regulators to keep up with new technology and promote innovation in this space, according to the article.
"We expect to see an increasing number of AI-based submissions in the coming years, starting with medical imaging devices, and we're working with experts in the field," he said.
Gottlieb noted that the FDA is working with experts to update the way the agency evaluates these new technologies during the approval process.