A proposal by the U.S. Food and Drug Administration (FDA) to eliminate 510(k) review for a number of artificial intelligence (AI) software applications is raising eyebrows in the radiology industry. The question is whether the proposal will be implemented after the Trump administration ends this week.
In a notice published January 15 in the Federal Register and attributed to Alex Azar, secretary of the U.S. Department of Health and Human Services (HHS), the FDA proposed to make permanent a number of regulatory changes introduced in April 2020 that were designed to provide regulatory relief to medical companies during the COVID-19 public health emergency.
One of these changes was the elimination of additional review for modifications made to medical imaging devices that had already received 510(k) clearance. At the time, the FDA said that it had become aware of modifications made to radiology devices to adapt them to the pandemic's unique conditions, such as altering mobile x-ray systems so patients can be imaged without entering a healthcare facility. Companies and healthcare providers should be able to use devices with such modifications without requiring them to undergo review, the agency said.
In the new document, the FDA states that an executive order issued in May 2020 directed the heads of all U.S. agencies to review regulatory standards that were rescinded temporarily during the pandemic to see if any "would promote economic recovery if made permanent." To that end, the FDA began reviewing whether the temporary waiver of 510(k) requirements issued early in the pandemic should be made permanent.
The Federal Register notice notes the high costs that the FDA's regulatory requirements impose on device and software developers. A 2010 study found that it cost $31 million to bring a low- to moderate-risk 510(k) product to market from conception to commercialization, with $24 million spent on FDA-dependent and/or related activities.
"These costs are barriers to new market entrants. To the extent imposing the section 510(k) premarket notification on a device does not create corresponding safety and efficacy benefits for Americans, those barriers are unjustified," the Federal Register document states. "Such barriers warrant scrutiny, particularly when market incumbents have an interest in retaining them."
The FDA's review focused on adverse event reporting of 221 unique types of medical devices, using the agency's Manufacturer and User Facility Device Experience Database (MAUDE). Ultimately, the agency identified 184 devices that could fall under the regulatory relief initiative, of which 10 were class I devices and 173 were class II. One device class was unclassified.
The review found that 60% of the 184 classes of devices under review had fewer than 100 reports in the MAUDE database over the past 10 years, and of these, 35 classes had no reports at all from 2010 to 2020. Another group of devices had no adverse events after the pandemic public health emergency, and an average of 1.5 events reported per year for the 10 years prior to the emergency declaration.
The Federal Register notice then goes on to note that the Federal Food, Drug, and Cosmetic Act that gives the FDA authority to regulate medical devices gives the agency the power to determine whether some types of class I devices no longer require 510(k) review to provide "reasonable assurance of safety and effectiveness" to the public.
Therefore, the agency suggests lifting the 510(k) requirement for the following classes of devices:
- High-level disinfection reprocessing instrument for ultrasonic transducers, liquid
- System, imaging, holography, acoustic
- Lung computed tomography system, computer-aided detection
- Chest x-ray computer-aided detection
- Computer-assisted diagnostic software for lesions suspicious for cancer
- Radiological computer-assisted triage and notification software
- Radiological computer-assisted detection/diagnosis software for fracture
- Radiological computer-assisted detection/diagnosis software for lesions suspicious for cancer
- Radiological computer-assisted prioritization software for lesions
- X-ray angiographic imaging based coronary vascular simulation software device
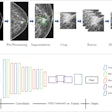
- Automated radiological image processing software
- Image acquisition and/or optimization guided by artificial intelligence
- Transducer, ultrasonic, obstetric
- Analyzer, medical image
- C-arm fluoroscopic x-ray system
- Coil, magnetic resonance, specialty
The FDA believes that eliminating 510(k) requirements for the devices on its list would save from $9.1 million to $364 million in startup costs if there were just one new product to enter the market in each device class, with more savings if more than one product is introduced.
"Instead of being costs passed along to patients and taxpayers, these savings could be invested in other areas such as research and development and manufacturing," the FDA states.
The FDA is inviting comments on the proposed rules on the federal government's electronic portal.
Many of the products that would receive regulatory relief fall under the realm of artificial intelligence, and experts in imaging AI are already expressing serious concerns about the proposal. For example, Dr. Hugh Harvey, managing director of U.K. consulting firm Hardian Health, notes that the FDA justified its decision to exempt radiology AI software due to an absence of adverse events over the past 10 years in the MAUDE database -- a move that ignores the fact that most AI applications are just now reaching the radiology market.
"So, my reaction is that this proposal is extremely dangerous and short-sighted," Harvey said in a January 16 tweet. "We cannot allow unregulated AI to make clinical decisions."
Other commentators, however, question whether the proposal will ever be implemented at all, given that there is only one day left in the Trump administration until President-elect Joe Biden is sworn into office on January 20. A Biden administration will undoubtedly take a more aggressive stance on regulating medical devices.
"This proposal was issued by HHS, not FDA," said Sam Surette, head of regulatory/quality at AI software developer Caption Health, in a tweet. "FDA had no role in this whatsoever, and certainly doesn't support it. Just some last-minute chicanery by Azar's HHS to undercut FDA further. There's no chance this proposal will actually be implemented under incoming administration."