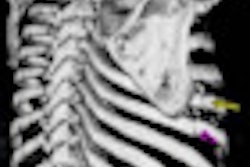
The U.S. Food and Drug Administration (FDA) has cleared new software from Riverain Technologies that improves the clarity of conventional chest x-ray images and allows radiologists to confirm the proper placement of medical devices, according to the company.
ClearRead +Confirm increases contrast and suppresses the clavicles and ribs in x-ray images, removing obstacles that could be obscuring the visibility of feeding, drug delivery and pain management tubes, lines, and cardiac wires, Riverain said.