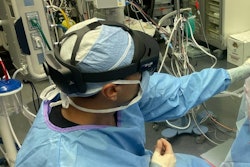
Novarad's VisAR technology recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for precision-guided intraoperative spine surgery.
The company said VisAR transforms patient imaging data into 3D holograms that can be viewed through an optical visor and superimposed onto patients, using CT fiducial markers for registration.
VisAR is available in the U.S., but Novarad said it anticipates usage in other countries in the coming months. The company also said head and neck surgical clearances are in the consideration phase with the FDA.