Connecticut-based software developer DeepLook announced it has received 501(k) clearance from the U.S. Food and Drug Administration (FDA) for software that automates segmentation and measurement of suspicious objects across all imaging modalities.
The DL Precise program is a one-click software tool that renders segmentation by contouring an object's margins and then measuring long- and short-dimension axes, area, and estimated volume. The tool eliminates manual measurements that require multiple mouse clicks and drags hundreds of times each day, the company said.
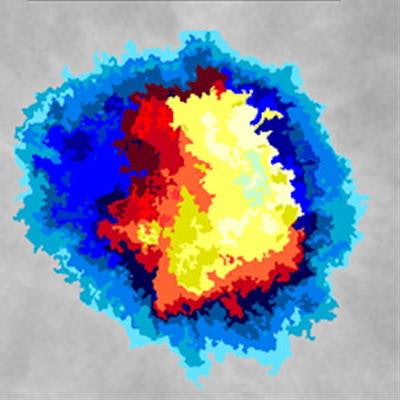
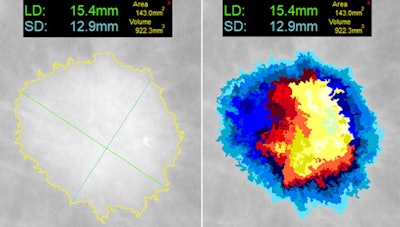 At left, one-click segmentation and measurement rendered by DL Precise. At right, DL Precise uses vivid color to illustrate segmentation. Image courtesy of DL Precise.
At left, one-click segmentation and measurement rendered by DL Precise. At right, DL Precise uses vivid color to illustrate segmentation. Image courtesy of DL Precise.DeepLook said DL Precise is the first of a series of medical imaging products it is developing to receive FDA clearance.