Interventional device developer Ethicon Endo-Surgery (EES) has launched a new global registry to collect the data of patients who undergo ablation with the company's Neuwave microwave ablation technology.
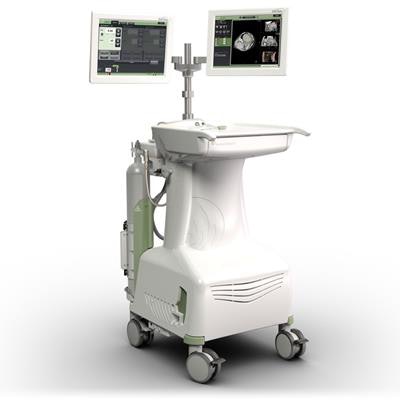
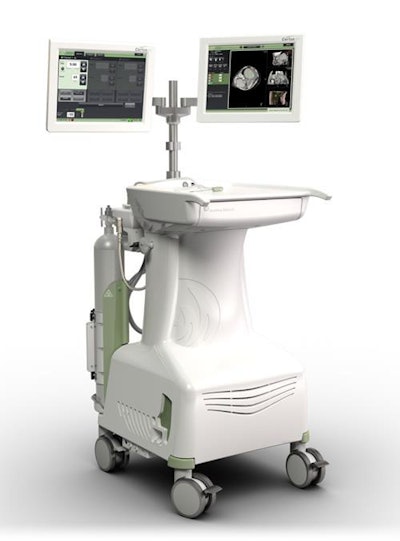 Neuwave microwave ablation machine. Image courtesy of Ethicon.
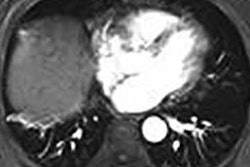
Neuwave microwave ablation machine. Image courtesy of Ethicon.The registry is set to include the information of approximately 1,500 patients with liver lesions being treated at 30 different centers during a five-year period, starting with the date of their first minimally invasive liver ablation procedure.
Ethicon's Neuwave technology allows clinicians to use heat, transmitted through image-guided probes, to destroy soft-tissue lesions, according to the company.
"Microwave ablation is an important treatment option that is increasingly being utilized throughout the world, and this data may provide new insights into factors that are critical for successful outcomes across a range of patients, clinical settings and healthcare providers," global registry consultant Dr. Paul Laeseke from the University of Wisconsin School of Medicine and Public Health said in a statement.