Ohio researchers used a prototype augmented reality system to overlay fluoroscopy images with a virtual needle for guided placement during interventional procedures, cutting dose significantly compared to conventional fluoro guidance, according to an article in Radiology.
The system overlaid existing conebeam CT data onto a real-time video to guide a needle to a spine target in pigs. Anatomic data were acquired before the procedure, and once it was finished, a single fluoro acquisition was used to check placement accuracy in the swine model.
The technique reduced overall radiation exposure by 37%, and by much more for the navigation component that the study focused on.
"The system was just as accurate as the gold standard, which is x-ray guided using 3D data from conebeam CT, and also the radiation exposure was reduced," said lead investigator Dr. John Racadio, professor of radiology and pediatrics at Cincinnati Children's Hospital Medical Center (Radiology, April 18, 2016).
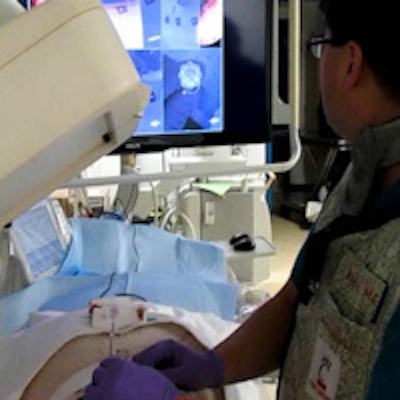
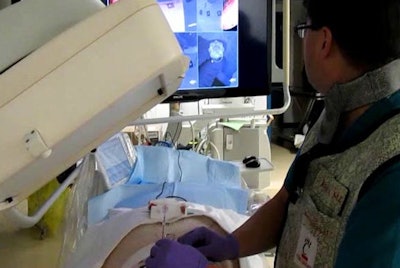 Operator uses virtual reality video guidance to advance the needle toward a target in the paraspinal muscles of a pig. Image courtesy of Dr. John Racadio.
Operator uses virtual reality video guidance to advance the needle toward a target in the paraspinal muscles of a pig. Image courtesy of Dr. John Racadio.Real-time imaging -- but at a high dose
C-arm conebeam CT can be used to collect 3D image data, enabling coregistration of fluoroscopic data with 3D images from any angle. Software then overlays fluoroscopic images of the needle navigation onto the conebeam CT images, the group wrote. Virtual graphics can be used to find the optimal skin entry point for the needle and connect it with the internal anatomic target point, with the graphics superimposed on the fluoroscopic image to monitor needle progress in real-time.
But the technique comes with two problems. First, both conebeam CT fluoroscopy and needle tracking expose the patient to radiation. Second, the patient must remain completely immobile to ensure that the CT data and virtual path graphics remain coregistered with anatomy.
These limitations can be overcome using a prototype augmented reality system set up to superimpose digital and virtual information onto "real world" needle guidance, the authors explained. Along with dose reduction, motion compensation using tracking markers on the patient can also be used to enable real-time registration of the conebeam CT and needle graphics to improve navigation.
The current study compared navigational accuracy and radiation dose during needle localization of targets with and without motion correction for virtual reality versus conebeam CT guidance in pigs.
In the study, three operators localized 2- to 4-mm bone fragment targets in the paraspinal muscles of nine Yorkshire pigs. Needle guidance was used to place 15 targets about 7-cm deep into the paraspinal muscles using an 11-gauge trocar. Fiduciary markers were placed to detect animal movement.
The prototype system was built with software and four small video cameras integrated within the frame of the x-ray detector of a C-arm (Allura, release 8.1; Philips Healthcare).
To acquire images, an operator rotated the C-arm to the CT fluoroscopy entry-point view, which superimposes a bull's-eye target on the entry point. Fluoroscopy images were then superimposed over the conebeam CT images and bull's-eye, and the operator positioned a hemostat over the bull's-eye graphic to mark the skin entry site.
In the guidance process that substituted virtual reality, the video entry-point view was used to determine the dermatotomy site by aligning the hemostat tip with the bull's-eye graphic. The rest of the process was the same, and each placement was followed by a confirmation conebeam CT image to check accuracy.
The study compared target depth, accuracy, and radiation dose based on dose-area product (DAP) for both procedures, testing the correlation between accuracy and target depth.
Same accuracy
The results showed no correlation between target depth and accuracy -- though accuracy might be expected to decline as the distance from skin to target increased. It did not, showing that use of the system is independent of operator experience in this model, Racadio and colleagues wrote. Adding motion control also did not change the accuracy.
What was significant were the differences in radiation dose. The radiation doses totaled 10.4 Gy · cm2 ± 10.6 for conebeam CT, 2.3 Gy · cm2 ± 2.4 for augmented reality, and 3.3 Gy · cm2 ± 4.6 for augmented reality with motion control (p < 0.05). The difference in total procedure dose showed similar distinctions.
The use of augmented reality, with or without motion correction, cut radiation dose "significantly while maintaining the accuracy of conebeam CT fluoroscopy," the authors wrote.
Conventional conebeam CT guidance has already shown substantial dose reductions compared to CT-guided procedures, and savings can be further enhanced with the use of virtual reality for needle positioning and advancement, benefiting both operators and patients, they noted.
Eighty-four percent of the radiation of the fluoro scan occurs in imaging the entry-point view, a step that is eliminated with augmented reality because needle positioning is performed using the video camera as the line of sight. The video procedure also allows the operator to insert the needle without having to worry about scatter radiation exposure to the body and hands.
"The biggest thing is having a system you can rely on for video guidance instead of x-ray, with decreased radiation exposure to the patient and obviously to the operator as well," Racadio told AuntMinnie.com.
Ergonomically, the augmented reality procedure was a lot easier to perform than the conventional fluoro guidance, he added.
"If I'm doing guided x-ray, I either have to use the needle holder or I have to advance the needle without fluoro and then take my hand away and use fluoro to readjust my needle back and forth," he said.
Getting rid of the cumbersome lead shield is another major advantage.
The fiduciary markers did add complexity to virtual reality. For motion correction to work, at least three markers must be visible by all four video cameras, but during needle advancement the operator's hands sometimes obscure them, the group wrote. The study was also limited by operators' knowledge of which system they were using.
Going forward, the system could transform the way many procedures are performed, Racadio said.
"We're looking at spinal surgery; we've actually done some human cadaver studies for spinal pedicle screw placement, and the results our outstanding," he said.
Of course, the closer one gets to anatomy that is in rapid motion, such as the heart, the more challenging it becomes, Racadio said.
The overall radiation dose reduction depends on what you measure, said study co-author Rami Nachabe, PhD.
"If you only look at the navigation, it's a 75% decrease in dose," Nachabe said. "If you take into account the 3D dataset you use to define your navigation path, then it's 50%. Thirty-seven percent is the decrease if you also take into account the final conebeam CT to evaluate your accuracy. But clinically speaking, you hardly need confirmation and you trust it, so the [overall] radiation dose would go down by 50%."
Nachabe is a clinical researcher and scientist at Philips, which built the prototype navigation system. Non-Philips co-authors had full control of the study data, the authors reported.