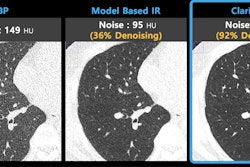
Artificial intelligence (AI) firm ClariPi has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its AI-based CT denoising technology.
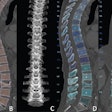
The company unveiled ClariCT.AI at RSNA 2018, showing that it uses a deep convolutional neural network trained with more than 1 million patient images to work in a vendor-neutral way to reduce noise and enhance image clarity for low-dose and ultra-low-dose DICOM CT images. The Clarity Engine is designed to separate image noise selectively while enhancing underlying structures, thus providing clarity-restored images.
ClariPi said the AI-based denoising technology potentially could enhance radiologists' reading confidence and enable accurate analysis for various imaging applications, including low-dose lung cancer screening CT.