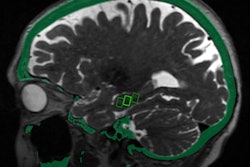
The U.S. Food and Drug Administration (FDA) has given clearance to researchers from the University of Maryland to proceed with a study that will investigate the use of MRI-guided focused ultrasound to open the blood-brain barrier.
The blood-brain barrier protects the brain, regulating the flow of substances in and out of it, according to a team led by Dr. Graeme Woodworth. But it also limits the ability of clinicians to deliver disease-fighting drugs to the brain, particularly in the case of brain tumors.
The trial will be conducted with patients undergoing surgery for glioblastoma at the University of Maryland Medical Center (UMMC) in Baltimore. Sponsored by InSightec, which developed the technology that will be used in the study, it will involve injecting microscopic inert gas-filled bubbles into a patient's bloodstream and then moving them back and forth with targeted sound waves to stretch the blood vessel walls and create temporary openings.
"If successful, this approach would allow us to use chemotherapy and other therapies in the brain in ways that are currently not possible," Woodworth said in a statement released by the university. "If we can selectively open the blood-brain barrier, then in the future we could give a much lower dose of powerful drugs, which would likely reduce toxic side effects and make treatments safer and more effective for patients."