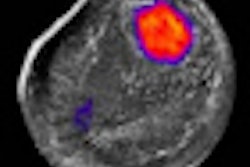
Emilio Quaia, MD, from the University of Trieste, will present the research in a Monday morning session at the RSNA meeting. Quaia and colleagues wanted to see if lesion enhancement patterns on contrast ultrasound could provide enough information on whether an incidental lesion was malignant to enable clinicians to make a diagnosis without biopsy.
The researchers began with a population of 25 patients with 55 noncirrhotic liver lesions that were considered indeterminate after contrast-enhanced CT or MRI, due to evidence of persistent hypovascular appearance or atypical enhancement patterns. They scanned each lesion with contrast-enhanced ultrasound after injection of a sulfur hexafluoride-filled microbubble contrast medium at different liver phases.
Two blinded independent readers reviewed contrast-enhanced cross-sectional images before and after analysis of contrast ultrasound cine clips, and they were asked to classify each lesion as malignant or benign based on contrast enhancement patterns.
Reviewing the cine contrast ultrasound clips improved the readers' accuracy: For reader 1, diagnostic accuracy improved to 85%, compared with 48% before ultrasound was reviewed; reader 2 improved to 87%, compared with 51% before ultrasound (p < 0.05).
The researchers found that specific lesion enhancement patterns, such as peripheral nodular enhancement in hemangiomas that appeared persistently hypovascular on CT and MRI, were telltale signs of malignancy.
The technique is already in clinical use in Europe, Quaia said, where ultrasound contrast is available for broad general applications. In the U.S., ultrasound contrast for applications outside the heart has not yet been approved by the U.S. Food and Drug Administration (FDA).