Aortic Rupture: (Pseudoaneurysm or Traumatic Aortic Laceration)
View cases of traumatic aortic laceration
Clinical:
Blunt traumatic aortic injury is classified on the basis of
the presence or absence of external wall abnormality as
significant aortic injury or minimal aortic
injury, respectively [24].
Significant aortic injury:
An aortic laceration is an intimal tear that varies in depth, with transmural extension (aortic rupture or transection) being the most severe form [11]. The adventitia is intact in about 60% of cases which aids in the development of a pseudoaneurysm that delays complete rupture [11]. It is estimated that 75-80% of thoracic aortic injuries are a result of high speed motor vehicle collision associated with rapid deceleration [4]. Traditionally, aortic laceration was felt to be secondary to sheering forces generated by abrupt thoracic deceleration at the points where the fixed aortic root and descending aorta join the mobile arch. More recently, it has been suggested that traumatic rupture may be caused by pinching of the aorta between the anterior and posterior components of the bony thorax when the chest is compressed [1]. Although head-on-collisions have historically been felt to place patients at the greatest risk for aortic injury, recent data suggests that side-impact collisions may be at greater risk [19]. Between 30% to 50% of patients with acute traumatic aortic injury will show no external sign of direct chest injury (ie: chest wall contusion or abrasion, or palpable rib/sternal fracture) [11].
Blunt trauma aortic injuries from motor vehicle accidents are more commonly associated with frontal collisions (60-65% of cases) than with lateral impact collisions [24]. Slightly fewer than 1% of all blunt trauma patients have a traumatic aortic laceration. However, for affected patients the prognosis is grave and between 80-90% of patients with traumatic aortic rupture die before emergency treatment can be instituted [11,19]. Of those that survive the injury, there is a 30% mortality within the first 6 hours [19] and 50% or more will die within 24 hours without repair [11,19]. For those who survive transport to a hospital, 80% live to be discharged if given appropriate treatment [18].
Tears tend to occur at sites of fixation such as: 1- The ligamentum arteriosum (aortic isthmus), just distal to or within 2 cm from the origin of the left subclavian artery, which is the most common site (95%); 2- the ascending aorta just beyond the aortic valve (5%) which is covered by pericardium and usually results in hemopericardium and death (incidence of ascending aortic injury is higher in autopsy series [20-25%]); and 3- the diaphragmatic hiatus (1% to 3%)- these injuries can be associated with diaphragmatic hernia in 10% of cases and with adjacent compression fractures of the thoracic spine [19]. Multiple aortic lacerations can be seen in 6% to 20% of cases [11]. Concomitant aortic branch vessel injury occurs in 4% to 10% of cases [11].
Treatment of aortic post-traumatic laceration:
1- Surgery: Surgery is generally emergent primary surgical repair. The mortality rate associated with emergent surgical treatment is 15-29%, in part due to the frequency of coexisting serious non-aortic injuries [6]. Post-operative paraplegia occurs in up to 25% of patients and is related to the duration of aortic cross-clamping [11] (the incidence of paraplegia is decreased with cardiopulmonary bypass and can be as low as 2.9% [19,23]). Mortality rates can be reduced when patients receive medical therapy during the acute phase of aortic injury with surgical repair postponed until the patient is stabilized [17].
2- Stent graft: Endovascular stent-grafts have also been used for the treatment of acute and chronic traumatic aortic aneurysms (although not yet approved for this use by the FDA [23]). Stent-grafts may be particularly useful in patients with contraindications to conventional surgery [17]. For successful endograft repair without covering the LSA, a proximal neck length from the injury to the origin of the LSA of 1.5-2 cm is preferred [23]. A higher rate of endograft failure has been noted with increasing acuity of aortic angulation [23]. This length is measured in a linear fashion in the sagittal plane, along the undersurface if the aortic arch and proximal descending aorta starting just distal to the origin of the LSA and terminating at the proximal end of the injury [23]. The aortic diameter should be measured 1cm proximal and 1 cm distal to the level of injury [23]. Cut-offs used for aortic diameter that are acceptable for endovascular repair range from a minimum of 18-20 mm to a maximum of 36-42mm [23]. Because the stent requires a 18-24 F delivery system, surgical exposure of the aorta or iliac vessels is sometimes required to obtain vascular access.
Complications include: paraplegia (the rate of paraplegia following endograft repair is up to 0.8% versus 3-19% following surgery [23]), obstruction of the take off of the left subclavian artery and perigraft leak/endoleak. [6] Type I endoleak is the most commonly reported and it typically occurs soon after treatment before healing of the pseudoaneurysm sac [23]. A shorter proximal neck length between the LSA and the injury has been shown to correlate with an increase in the prevalence of type Ia endoleak (likely a result of poor graft apposition) [23]. Type II endoleaks can occur as a result of flow via the LSA, bronchial arteries, and intercostal arteries [23]. Endograft collapse (partial or complete) is a serious potential complication- an increased risk is associated with a proximal neck length of less than 2 cm and an acute angulation of the aortic arch (less than 90?) [23]. A focal collapse may appear as a focal concavity in the endograft [23]. Partial or complete collapse will appear as narrowing or irregularity of the endograft lumen with blood flow diverted between the aortic wall and endograft [23]. Part of the problem is related to the use of grafts designed for the straight course of the abdominal aorta [23]. The curved proximal descending aorta can predispose for poor stent graft apposition- referred to as the "bird's beak effect"- in which the inferior proximal end of the graft projects into the aortic lumen along the concave surface of the inferior aortic arch creating a nidus for endoleak or stent collapse [23].
Endograft infection can occur in about 5% of patients [23]. In the abdominal aorta, more than 5 mm of perigraft soft tissue between the aneurysm wall and the graft is abnormal and may suggest infection [23].
The left internal mammary artery originates from the LSA [23]. In patients with LIMA bypass grafts, LSA occlusion can result in myocardial ischemia [23].
The mortality rate after endograft repair for acute aortic tear is 4-9.4%, is substantially lower than that after surgery (8-24%) [23]. There is little long term data on the outcome following endovascular stent repair of acute aortic injury [19].
About 2% to 5% of patient with untreated traumatic aortic injury survive and develop a chronic pseudoaneurysm- most commonly in the region of the ligamentum arteriosum [9,11]. Rupture can occur in up to 25% of chronic pseudoaneurysms [6]. Rupture can be fatal- 10 year survival for those who decline surgical repair is 66%, but is 85% for those who undergo treatment. [2]
Pediatric patients: Traumatic aortic injury is uncommon in
pediatric patients accounting for only 2.1% of pediatric trauma
deaths [22].
Minimal aortic injury: [24]
The incidence of minimal aortic injury (MAI) is estimated to be
10-30% of all blunt trauma that manifests at imaging [24]. MAI is
defined as a sub-centimeter intimo-medial abnormality (thrombus or
flap) without an associated external contour deformity [24]. A
localized intramural hematoma without an external contour
abnormality has also been accepted as a manifestation of MAI [24].
A restrained frontal collision with the use of seat belts and
airbag deployment is more often associated with MAI [24].
MAI can be treated with non-operative management consisting of
blood pressure and heart rate control [24]. Spontaneous healing of
MAI can occur in the first 4-8 weeks after injury and only 10-15%
of cases persist or progress at followup [24].
However, dislodgement of intimal thrombi associated with MAI with
downstream embolization has been described with the presence of
new renal or splenic infarcts in up to 17% of MAI patients [24].
X-ray:
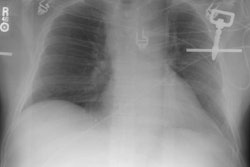
Plain films: The greatest value of the frontal chest radiograph is not in suggesting aortic injury, but in excluding it. A truly normal frontal CXR has a 98% negative predictive value for aortic injury [9,10] (96% for a supine view). However, others report a normal or deceptively underwhelming CXR in up to 7% of patients with aortic injury [19]. Other authors indicate that a mediastinal abnormality on CXR has a diagnostic sensitivity of 90-95% for thoracic aortic injury, but a specificity of only 5-10% [20].
Unfortunately, the specificity of an abnormal exam is poor [11]. In fact, the incidence of aortic rupture in patients evaluated by angiography solely because of an abnormality found on the frontal CXR, is only between 10-20%.
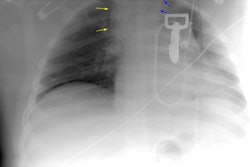
Plain film findings which suggest aortic disruption include (an upright frontal film is essential, but unfortunately difficult to obtain in trauma patients): 1- Transverse width of the mediastinum greater than 8 cm just above the aortic knob; 2- Mediastinum to chest width ratio greater than 25% ; 3- Aortic contour abnormality with blurring of the outline of the descending aorta; 4- Aortopulmonary window opacification; 5- Widened left paraspinal stripe; 6- Depression of the left mainstem bronchus more than 40 degrees below the horizontal; 7- Tracheal deviation to the right of the T4 spinous process; 8- NG tube deviation to the right at the T4 spinous process; 9- Right or left pleural cap; 10- Left hemothorax without rib fracture; 11- Right paratracheal stripe thicker than 5mm; 12- First rib fracture is also a marker of severe chest trauma and up to 18% of patients with aortic tears are also noted to have a fracture of the first rib [2]. Mediastinal widening is the most frequent finding on CXR, but it is not the most sensitive finding [19]. More discriminating findings include any abnormality of the transverse arch or loss of the aortopulmonary window [19].
Computed Tomography:
CT is less invasive, readily available in most hospitals at all
hours, and can result in substantial cost savings when evaluating
patients for suspected aortic injury [16]. CT scans should not
be performed in hemodynamically unstable patients, in patients
with obvious plain film radiographic evidence of aortic injury
(unless the surgical team will accept the CT findings and not
require further angiographic evaluation), or in patients in whom
the mechanism of injury strongly suggests aortic injury
(controversial- some authors would screen with CT). These patients
should instead proceed directly to aortography. In the absence of
these criteria, CT can be used as a screening tool, especially in
those patients undergoing CT examination for other injuries [4,5].
A normal CT scan essentially excludes aortic injury (ie: negative predictive value approach 100%)- fewer than 1% of patients with a normal CT are found to have an aortic rupture on subsequent aortography [3,16]. 64 MDCT has a reported sensitivity of 96-98%, specificity of 99.8%, PPV of 92.3%, NPV of 99.9%, and an accuracy of 99.8% [21]. Previously, CT had been reported to have a lower specificity because the scan findings are completely normal in only about 25% of patients. Previously, the false positive rate for CT in the detection of aortic injury had been reported between 0-39% [10,16]. This is because the indirect sign of periaortic hematoma is associated with a high rate of false-positive results [21].
The accuracy of CT for the detection of branch vessel injury has not been extensively studied.
Exam: The scan should begin at the level of the diaphragm and progress cephalad to above the aortic arch. Other authors recommend beginning imaging at the base of the neck and extending inferiorly to the diaphragm [11]. A right arm injection is preferred for contrast injection to decrease streak artifacts that occur as the contrast bolus traverses the thoracic inlet vessels [16]. Parasagittal reformatted left anterior oblique images can be created to evaluate the arch for contour abnormalities [10].
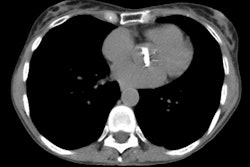
Findings: Direct findings on CT of aortic transection include irregular contour or a change in caliber of the aortic lumen at the level of the isthmus (within 2 cm of the origin of the left subclavian artery) [19]- often a focal outpouching consistent with a pseudoaneurysm can be seen in association with this finding. Isthmic injuries are seen most commonly along the medial curvature of the arch at the level of the left main pulmonary artery and left main bronchus [19]. An intralumenal flap appears when the wall of the aorta is outlined by contrast material because of a contained rupture [19]. Direct signs of aortic injury on MDCT do not require confirmation with angiography [21].
The presence of a mediastinal hemorrhage is considered indirect
evidence of an aortic transection. The hematoma associated with
aortic injury is usually periaortic and may extend inferiorly
along the descending aorta. The hemorrhage may appear as streaky
increased density within the mediastinal fat or be a more focal
collection of blood. However, up to 17% of aortic tears can occur
without significant mediastinal hematoma [9]. Some authors would
feel that if a mediastinal hematoma is present, the patient should
undergo aortography regardless of the appearance of the aorta on
CT [9]. Unfortunately, the presence of a mediastinal hematoma does
not confirm the diagnosis of aortic transection as it may also be
seen in blunt trauma patients for other reasons such as sternal or
thoracic spine fracture, or venous bleeding (ie: the finding is
very sensitive, but not specific for aortic injury). In fact, up
to 97% of angiograms performed on patients with only mediastonal
hematoma, but no aortic abnormality, are negative [10]. Some
authors now recommend that patients with isolated, small
mediastinal hematomas separate from the aorta and a normal
appearing aorta at helical CT do not require angiographic
evaluation [12-15,19]. Instead, these patients can be followed
clinically and with plain film radiographs for a 4 to 6 month
period [12].
Difficulties in exam interpretation may occur secondary to normal
thymus (mimics hematoma), the pulmonary artery, periaortic
atelectasis (mimics periaortic hematoma), motion (pulsation
artifact), or partial volume effects [11]. Cardiac gating permits
more accurate evaluation of the aorta, but multiplanar reformated
images can be degraded by a "band artifact" [20].
Findings that can help differentiate hemorrhage from other
sources (such as small mediastinal veins or small branch arteries)
versus associated hemorrhage associated with aortic injury include
a preserved fat between the hemorrhage and the intact aortic wall
and hemorrhage without significant mass effect [24].
Minimal aortic injury:
Minimal aortic injuries (MAI) affecting only the intima of the vessel wall are estimated to occur in 10% of patients with acute traumatic aortic injury [19]. MAI is most often seen at the aortic isthmus [24]. The two most common findings of intimal tear in MAI are a rounded or triangular filling defect 10 mm or less (80% of cases) or a thin focal intimal flap attached to the vessel wall without an external contour deformity (seen in 15% of cases) [24]. A small (1 cm or less in thickness) intramural hematoma represents a grade II injury, but is considered to be in the spectrum of MAI and constitutes 5% of all MAI cases [24]. Intramural hematoma appears as increased attenuation in the aortic wall due to hemorrhage from ruptured vasa vasorum or small intimomedial tears [24]. MAI can also appear as a periaortic hematoma without other direct finding of aortic injury [19]. Subsequent short term followup imaging can demonstrate small intralumenal thrombi at the site of the previously occult injury [19]. These lesions pose a substantial problem because they are being encountered with an increased frequency, the lesions are often not seen on conventional angiography or intravascular US [19].
Angiography: Angiographic findings of thoracic aortic injury include the identification of a pseudoaneurysm, intimal or media tear, or rupture with contrast extravasation. Subtle lesions may be missed if two views (lao and ap) are not obtained. False positive exams occur in approximately 1% of cases [11]. A congenital ductus diverticulum may cause a false positive exam. Findings which help to identify a ductus diverticulum are its smooth contour, obtuse angles with the aortic wall, and the absence of overlapping density at its edges.
Transesophageal echocardiography (TEE): TEE can be used in the evaluation of aortic injury. Direct signs of aortic injury include depiction of an aortic flap or pseudoaneurysm, or an aortic wall hematoma [11]. Atherosclerotic disease can produce false-positive exams. Findings which suggest the presence of a mediastinal hematoma include a distance of more than 3 mm between the probe and the aortic wall; a double contour of the aortic wall; or an "ultrasound signal" between the aortic wall and visceral pleura [11]. Overall data from recent studies suggest a sensitivity of 57-63% and a specificity of 84-91%. The exam is technically inadequate in about 15% of patients, is invasive, requires technical skill, and has a 2.9% complication rate [11].
REFERENCES:
(1) Semin Ultrasound CT MR 1996 Apr;17(2):119-141
(2) Radiographics 1997; 17: 27-45
(3) J Thorac Imag 1996; 11: 39-45
(4) AJR 1994; 162(5): 1047-1052
(6) Radiology 1997; 205: 657-662
(8) AJR 1998; Wong YC, et al. Periaortic hematoma on helical CT of the chest: A criterion for predicting blunt traumatic aortic rupture. 170: 1523-1525
(9) Radiographics 1998; van Hise ML, et al. CT in blunt chest trauma: Indications and limitations. 18: 1071-1084
(10) Radiographics 1998; Kuhlman JE, et al. Radiographic and CT findings of blunt chest trauma: Aortic injuries and looking beyond them. 18: 1085-1106
(11) Radiology 1998; Patel NH, et al. Imaging of acute thoracic aortic injury due to blunt trauma: A review. 209: 335-348
(12) Society of Thoracic Radiology Course Syllabus 1999; Fishman JE. Imaging of aortic and great vessel trauma. p. 75-84
(13) Society of Thoracic Radiology Course Syllabus 1999; Primack SL. CT in blunt chest trauma: Indications and limitations. p. 87-98
(14) AJR 1999; Fishman JE, et al. Direct versus indirect signs of traumatic aortic injury revealed by helical CT: Performance characteristics and interobserver agreement. 172: 1027-1031
(15) Radiology 1999; Dyer DS, et al. Can chest CT be used to exclude aortic injury? 213: 195-202
(16) AJR 2001; Parker MS, et al. Making the transition: The role of helical CT in the evaluation of potentially acute thoracic aortic injuries. 176: 1267-1272
(17) AJR 2002; Fattori R, et al. Indications for, timing of, and results of catheter-based treatment of traumatic injury to the aorta. 179: 603-609
(18) AJR 2007; Sammer M, et al. Indeterminate CT angiography in blunt thoracic trauma: is CT angiography enough? 189: 603-608
(19) Radiology 2008; Steenburg SD, et al. Acute traumatic aortic injury: imaging evaluation and management. 248: 748-762
(20) Radiographics 2008; Kaewlai R, et al. Multidetector CT of blunt thoracic trauma. 28: 1555-1570
(21) AJR 2008; Steenburg SD, Ravenel JG. Acute traumatic thoracic aortic injuries: experience with 64-MDCT. 191: 1564-1569
(22) AJR 2010; Pabon-Ramos WM, et al. Radiologic evaluation of blunt thoracic aortic injury in pediatric patients. 194: 1197-1203
(23) Radiographics 2010; Morgan TA, et al. Acute traumatic aortic
injuries: posttherapy multidetector CT findings. 30: 851-867
(24) Radiorgaphics 2020; Kapoor H, et al. Minimal aortic injury:
mechanisms, imaging manifestations, natural history, and
management. 40: 1834-1847