Congestive Heart Failure
Clinical:
Congestive heart failure is a complex clinical syndrome characterized by dyspnea, fatigue, and fluid retention which results from any structural or functional cardiac disorder that impairs the ability of the LV to fill with or eject blood [11]. (CHF) is a debilitating and common condition affecting 6-10% of the elderly population in developed countries [1]. Approximately 550,000 new cases are diagnosed annually [6]. Patients with chronic LV dysfunction are at increased risk for adverse cardiovascular outcomes [8]. Heart failure is the leading cause of morbidity, mortality, and hospitalization in patients older than 60 years of age (heart failure accounts for about 20% of all hospital admissions among patients over 65 years [8]) [3]. In North America, the most common cause of CHF is ischemic heart diease (CAD is the underlying etiology for heart failure in 70% of cases [6,8]) [1]. Patients with heart failure and CAD also fare worse than patients with CHF unrelated to CAD [9].
The pathologic remodeling associated with CHF also involves a
shift toward glycolytic metabolism and downregulation of
fatty-acid oxidation, disorganization of the sarcomere,
alterations in calcium handling, changes in contractility, loss of
myocytes with fibrotic replacement, LV dilatation, systolic and
diastolic dysfunction, and electrical disturbances with a
propensity to malignant ventricular arrhythmia [11]. Sympathetic
nervous system dysfunction also plays a role in the evolution of
CHF. Under normal circumstances sympathetic activation results in
an increased HR, a more forceful contraction, and enhanced
atrioventricular conduction, while the parasympathetic system has
its primary effects on HR regulation [8]. In patients with HF,
there is an increased sympathetic drive to the myocardium- mainly
the result of local defects in norepinephine synthesis, storage,
and release [8]. Defects include a decreased cardiac NE uptake
(impaired reuptake of norepinephrine from the neural cleft back
into the presynaptic neuron) and reduced expression of cardiac NE
transporter (a result of post-transcriptional downregulation)
[8,10]. As a consequence a higher concentration of NE is present
in the sympathetic cleft (and in circulating norepinephrine
levels), leading to chronic stimulation and down-regulation of
myocardial beta-adrenoreceptors [8,10]. Loss of normal sympathetic
control over myocardial function is associated with an increased
incidence of adverse outcomes (such as unstable arrhythmias) [8].
Reduction of sympathetic nervous system activity is an important
target for drug treatment in HF patients [11].
No all patients with heart failure have decreased LVEF and these
patients can account for approximately one-half of heart failure
patients [15]. Heart failure with preserved EF (HFpEF) is
functionally characterized by impaired LV relaxation, increased LV
stiffness, and elevated LV filling pressure [15]. Patients with
HFpEF have been shown to have reduced myocardial sympathetic
innervation on PET imaging [15].
The long term prognosis for patients with heart failure is poor
with a 5-year mortality of 59% for men and 45% for women [2]. The
prognosis is generally worse for CHF patients with CAD compared to
those without CAD [6]. Patients with ischemic heart failure and
depressed LVEF (less than 35%) have an increased risk of
arrhythmic death [14]. Approximately 50% of heart failure deaths
are caused by ventricular tachycardia [3]. Routine implantation of
ICD's in patients with a prior MI and an ejection fraction of less
than 30% has been shown to reduce mortality from 20% to 14% [3].
The presence, extent, and characteristics of myocardial scar
assessed with contrast-ehnamced MRI have incremental prognostic
value over LVEF to predict the occurence of VT/VF [14]. On DCE MR,
arrhythmic risk increased significantly when the scar mass
exceeded 1.4-5% of the LV volume (with the risk reaching a plateau
at larger levels of scar mass) [14].
Left bundle branch block (LBBB) can be found in about 25% of affected patients and has been reported to be associated with increased disease severity and mortality [1]. Cardiac resynchronization therapy has been shown to improve left ventricular (LV) function and reduce hospitalization and death in patients with advanced/chronic CHF and LBBB [1]. The current selection criteria for CRT are an LVEF < 35% and a widened QRS complex (>120 ms) [4,5]. CRT can improve clinical manifestations and quality of life, reduce hospitalizations for CHF, reduce complications, and risk of death (increase survival) [4,5]. Unfortunately, between 20-50% of patients do not respond to CRT [1,5]. Lower MIBG uptake (H/M ratio below 1.36) is associated with a higher likelihood for lack of response to CRT [4]. The lower MIBG uptake may reflect hearts with more severe myocardial damage that are less likely to respond to CRT [4]. Following successful CRT intervention, there is improved cardiac uptake of MIBG [4].
A widened QRS complex is indicative of electrical dyssynchrony, however, the presence of left ventricular mechanical dyssynchrony suggests patients that are more likely to respond to CRT [5]. Phase image analysis from gated cardiac SPECT examinations can be used to evaluate for the presence of LV dyssynchrony [5]. One added benefit is that additional prognostic information can also be obtained from the perfusion images [6]. Dyssynchrony can also be evaluated using tri-plane tissue Doppler imaging (TDI) to measure segmental myocardial velocities [6]. LV dyssynchrony of more than 65 milliseconds on tri-plane tissue Doppler imaging can also predict CRT response [6]. TDI is highly operator dependent, and 20% of patients have a suboptimal acoustic window [7].
Extensive LV scarring - or scar in the region in which the LV pacing lead is positioned (typically the posterolateral region)- can also result in a decrease likelihood for response to CRT (extensive scarring is predictive of lack of CRT response with a sensitivity of 83% and a specificity of 74%) [5]. This suggests that improvement in LV function is prohibited in the presence of extensive scar tissue [5].
Surgical revascularization can result in improved patient survival in patients that are shown to have myocardial viability.
X-ray:
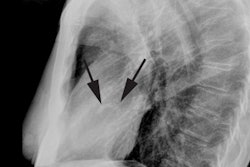
CXR: Vascular redistribution with relative hypervascularity of
the upper lung zones indicates a PCWP greater than 13 but less
than 18 mmHg; interstitial edema is seen at PCWP of 18-25 mm Hg;
and alveolar edema at PCWP of greater than 25 mm Hg [12].
Interstitial edema results in loss of delinieation of subsegmental
and segmental vessels, thickening of the interlobular septa
(Kearly B lines), and peribronchial cuffing [13]. Thickening of
the interlobar fissures and subpleural effusions may also occur
[13]. Further increases in capillary pressure lead to alveolar
edema/coalescent alveolar opacities [13]. In more chronic disease,
enlarged mediastinal lymph nodes can be seen due to concurrent
lymphatic fluid overload [13].
Nuclear medicine: Patients with chronic heart failure demonstrate reduced uptake of 123I-MIBG. See "Adrenergic cardiac imaging" for more details.
REFERENCES:
(1) J Nucl Med 2006; Thompson K, et al. Is septal glucose metabolism altered in patients with left bundle branch block and ischemic cardiomyopathy. 47: 1763-1768
(2) J Nucl Med 2007; Schinkel AFL, et al. Assessment of myocardial viability in patients with heart failure. 48: 1135-1146
(3) Radiology 2007; Wu JC, et al. Cardiovascular molecular imaging. 244: 337-355
(4) J Nucl Cardiol 2007; D'Orio Nishioka SA, et al. Cardiac sympathetic activity pre and post resynchronization therapy evaluated by 123I-MIBG myocardial scinitgraphy. 14: 852-859
(5) J Nucl Med 2007; Henneman MM, et al. Nuclear imaging in cardiac resynchronization therapy. 48: 2001-2010
(6) J Nucl Cardiol 2008; Chen J, et al Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. 15: 127-136
(7) J Nucl Med 2007; Henneman MM, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? 48: 1104-1111
(8) J Nucl Cardiol 2009; Jacobson AF, et al. 123I-mIBG scintigraphy to predict risk for adverse cardiac outcomes in heart failure patients: design of two prospective multicenter international trials. 16: 113-121
(9) J Nucl Cardiol 2009; Soman P. Gated SPECT myocardial perfusion scinitgraphy: a multi-faceted tool for the evaluation of heart failure. 16: 173-175
(10) J Nucl Cardiol 2009; Gerson MC, et al. Of fight and flight. 16: 176-179
(11) J Nucl Cardiol 2009; Flotats A, Carrio I. Radionuclide
noninvasive evaluation of heart failure beyond left ventricular
function assessment. 16: 304-315
(12) AJR 2012; Barbosa EJM, et al. Current role of imaging in the
diagnosis and management of pulmonary hypertension. 198: 1320-1331
(13) AJR 2013; Nemec SF, et al. Lower lobe-predominant diseases
of the lung. 200: 712-728
(14) J Nucl Med 2015; Landau SM, et al.
Measurement of longitudinal beta-amyloid change with 18F-Florbetapir PET and standardized
uptake value ratios. 56: 567-574
(15) J Nucl Med 2017; Aikawa T, et al.
Impaired myocardial sympathetic innervation is associated with
diastolic dysfunction in heart failure with preserved ejection
fraction: 11C-hydroxyephedrine PET study. 58:
784-790