Myocardial Bridging
Clinical:
Myocardial bridging is felt by some to represent the most common congenital coronary abnormality [12]. A myocardial bridge can be described as partial or complete depending on the extent to which the coronary segment is surrounded by myocardium [12]. A parital myocardial bridge is one in which more than 75%, but less than 100% of the artery is encased by myocardium [12]. In a complete myocardial bridging the coronary artery dips below the epicardium, is completely encased by myocardium, and runs within the myocardium for a short distance [1,12]. During systole, this segment is compressed [3] and may show up to 50% systolic narrowing [9]. This is generally not clinically significant because most coronary flow occurs during diastole [9]. However, at rapid heart rates a reduction in coronary blood flow has been observed [4]. Since the LCX and RCA follow the AV groove, myocardial bridging is seen almost exclusively with the LAD- most commonly in the middle segment, followed by the distal LAD [2,12]. The affected portion of the vessel can be covered by a varying amount of myocardium. It has been proposed that a segment covered by 2-2.3 mm or more of myocardium should be considered a "deep" segment [5,12]. Myocardial bridging is a rare angiographic finding (0.5-2.5% of patients at angiography [2]), but the reported incidence at pathologic analysis is higher (15-85%) [1,2]. This discrepancy is likely due to the fact that many affected patients are asymptomatic [2]. On MDCT imaging, myocardial bridging can be identified in 3.5-26% of patients (some even suggest up to 44% of patients [12]) [5,6,7].
Myocardial bridging is most commonly and incidental asymptomatic
finding associated with an excellent rate of 97% at 5 years, but
certain characteristics may be associated with cardiovascular
morbidity
[12]. Longer and deeper bridges and those that exhibit greater
degrees
of systolic compression (over 70%) are more common in symptomatic
patients [12]. Myocardial bridging has been reported to be
associated
with angina,
myocardial ischemia (particularly if the segment is associated
with a
coexistent stenosis in the vessel), myocardial infarction,
arrhythmias,
and sudden death [1,3,9]. The condition can also pose a problem
during
cardiac bypass surgery [3].
Several mechanisms have been postulated to
explain ischemic resulting from myocardial bridging including
vascular
compression during systole with resultant lumenal narrowing,
delayed
diastolic relaxation (and hence delayed initiation of diastolic
blood
flow), and increased contractility [6,10,11]. With increasing
systolic
compression (to levels greater than 75%), the lumenal obstruction
may
persist into diastole and this may result in impaired coronary
flow
reserve during periods of tachycardia (due to diastolic
shortening)
[12].
Some authors
also feel that myocardial bridging predisposes to the development
of
atherosclerosis in the coronary segment proximal to the bridge due to
increased shearing forces (the
bridged coronary artery segment is frequently spared from
atherosclerotic
disease)- hence, bridging may be a risk factor for CAD
[5,9,10,12,13].
Treatment in symptomatic patients consists of beta-antagonists (blockers), calcium antagonists (channel blockers), and antiplatelet agents [4,6]. Beta-blockers reduce tachycardia and increase diastolic coronary filling time with a decrease in myocardial contractility and compression of the coronary arteries [12]. Nitrates should generally be avoided because they increase systolic compression and may lead to worsening of the clinical symptoms [6]. Surgical myotomy or bypass surgery also have good success, but because they are more invasive these treatments should be reserved for patients that are refractory to medical management [4].
X-ray:
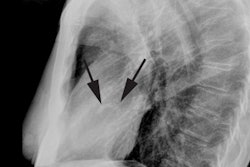
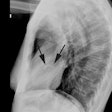
The characteristic angiographic finding is systolic narrowing of the LAD that disappears during diastole.
On perfusion SPECT imaging, exercise stress-induced perfusion defects can be found in 21-88% of patients with myocardial bridging [1,4,9]. In general, the greater the degree of vascular compression (systolic narrowing), the greater the likelihood for perfusion defects [1,8,11]. Perfusion abnormalities can be identified in up to 43% of patients with systolic narrowing of 50% or greater [11] (stress induced defects can be seen nin up to 63% of patients with angiographic compression >50%, 35% of patients with 50% systolic compression, and in 0% of patients with <50% systolic compression [12]). Myocardial bridging that produces a 75% or greater systolic stenosis can demonstrate reversible perfusion defects even when pharmacologic stress imaging is used [1]. Lesser degrees of narrowing are associated with a lower incidence of perfusion abnormalities [1] and in one study, were not seen in patients with less than 50% systolic compression [11]. Despite the fact that coronary artery flow occurs predominantly during the diastolic phase, reversible perfusion abnormalities are strongly associated with the degree of systolic vessel narrowing suggesting that the compression must persist into a portion of diastole [8]. The length of the tunneled vessel does not appear to affect the presence of absence of perfusion abnormalities on MPI [8].
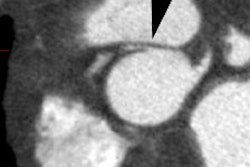
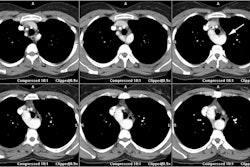
On MDCT, myocardial bridging can be found in up to 3.5-26% of
patients [3]. Bridging is best identified on sagittal
reconstruction
images [3]. The degree of systolic compression seems to correlate
with
the depth of the tunneled segment, but not the length [7].
Intravascular ultrasound can demonstrate systolic compression of
the
involved epicardial segment and the presence of a surrounding
echolucent muscle band surrounding the artery [10]. Intravascular
Doppler can accurately assess the hemodynamic effects of
myocardial
bridging by demonstrating abrupt early diastolic flow
acceleration-
termed "finger tip" phenomenon, a rapid mid-diastolic
deceleration,
mid-to-late diastolic plateau phase, and a retrograde systolic
flow
pattern [10].
REFERENCES:
(1) J Nucl Cardiol 2005; Vallejo E, et al. Myocardial perfusion SPECT imaging in patients with myocardial bridging. 12: 318-323
(2) Radiographics 2006; Kim SY, et al. Coronary artery anomalies: classification and ECG-gated multi-detector row CT findings with angiographic correlation. 26: 317-334
(3) AJR 2006; Kantarci M, et al. Detection of myocardial bridging with ECG-gated MDCT and multiplanar reconstruction. 186: S391-394
(4) J Nucl Cardiol 2006; Sagar S, et al. Ischemia on "resting" SPECT myocardial perfusion images in a patient with myocardial bridging. 13: 119-122
(5) AJR 2007; Zeina AR, et al. Myocardial bridge: evaluation on MDCT. 188: 1069-1073
(6) AJR 2007; Harirolan T, et al. Myocardial bridging on MDCT. 188: 1074-1080
(7) Radiology 2008; Leschka S, et al. Myocardial bridging: depiction rate and morphology at CT coronary angiography- comparison with conventional coronary angiography. 246: 754-762
(8) J Nucl Cardiol 2011; Tang K, et al. The role of myocardial
perfusion imaging in evaluating patients with myocardial bridging.
18:
117-122
(9) AJR 2011; Young PM, et al. Cardiac imaging: part 2, normal,
variant, and anomalous configurations of the coronary vasculature.
197:
816-826
(10) J Nucl Cardiol 2011; Gakbraith EM, et al. SPECT perfison
imaging and myocardial bridges: bridging the gap of diagnostic
uncertainty. 18: 1000-1002
(11) J Nucl Cardiol 2011; Gawor R, er al. Myocardial perfusion
GSPECT imaging in patients with myocardial bridging. 18: 1059-1065
(12) J Cardiovasc Comput Tomogr 2012; Nakanishi R, et al.
Myocardial
briding on coronary CTA: an innocent bystander or a culprit in
myocardial infarction? 6: 3-13
(13) J Cardiovasc Comput Tomogr 2012; Pursnani A, et al. Coronary CTA assessment of conrary anomalies. 6: 48-59