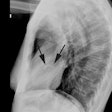
Aortic Stenosis:
View cases of Aortic Stenosis
Clinical:
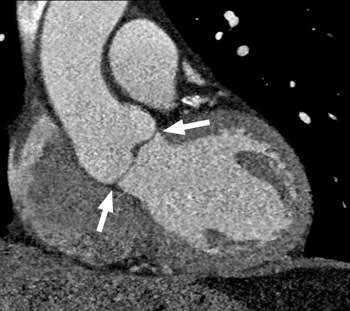
Etiologies of aortic stenosis include unicuspid / bicuspid aortic valve, rheumatic heart disease, and senile degenerative change of a tricuspid valve. Subvalvular aortic stenosis can occur as a result of asymmetric septal hypertrophy (IHSS).
Bicuspid Valve: A bicuspid
aortic valve occurs in about 0.5% to 1% of the population [4]
(M>F 3-4:1) and the valve is subject to premature degeneration.
A bicuspid valve is the most common cause of isolated aortic
stenosis.On average, stenosis occurs in patients with a BAV about
a decade earlier than in patients with a tricuspid valve [12].
Rheumatic Heart Disease:
Isolated aortic stenosis is uncommon from rheumatic heart disease
and these patients almost always have associated mitral valve
disease. Deposition of calcium in the valve is usually
minimal.Worldwide, rheumatic heart disease accounts for most cases
of aortic stenosis [12].
Clinical findings in aortic stenosis include shortness of breath, angina (in the absence of coronary artery disease), syncope (usually with exercise due to hypoperfusion), decompensated heart failure, and sudden death (as a result of dysrythmia). Concomitant coronary artery disease is common in patients with aortic stenosis, and significant CAD is found in 20-50% of severe AS patients [29].
A normal valve area is considered between 3 to 4 cm2
[2]. Narrowing of the orifice to half its normal size produces
almost no hemodynamic abnormality [2] and most patients are
asymptomatic until the stenosis reduces the the aortic valve area
to approximately 1 cm2 [8]. However, further narrowing
to about one-quarter normal size becomes hemodynamically
significant [5]. Mild aortic stenosis is present with a valve area
between 1.5-2 cm2; moderate stenosis with an area of
1.0-1.5 cm2; severe stenosis with a valve area of
0.7-1.0 cm2; and critical stenosis is present when the
valve area is 0.7 cm2 or less [2,5]. Other criteria for
severe stenosis include a mean transvalvular pressure gradient
> 40 mm Hg, or if the jet velocity exceeds 4.0 m/s [9]. A mean
pressure gradient of less than 20 mm Hg is mild and between 20-40
mm Hg is moderate stenosis [26].
When the valve orifice narrows to this degree a significant pressure gradient develops at resting flow rates which places an increased pressure load on the left ventricle [12]. The pressure overload leads to left ventricular hypertrophy to accomplish the increase workload required by the ventricle [2,12]. As hypertrophy develops, coronary blood flow reserve becomes limited [2]. Another consequence of hypertrophy is the development of diastolic dysfunction due to the greater filling pressure required to fill the hypertrophied left ventricle [2]. This increased filling pressure is transmitted back to the lungs where it can cause symptoms of pulmonary congestion [2]. In patients with severe stenosis and substantial LVH, irreversible myocardial fibrosis eventually develops [12]. Fibrosis typically begins in the subendocardium and impairs longitudinal ventricular contraction [12]. Delayed myocardial enhacement on MR appears to be prevalent in patients with aortic stenosis and a LV wall thickness exceeding 18mm [12]. The delayed enhancement pattern has been described as patchy and subendocardial, with a propensity to invovle the basal segments where wall stresses are highest [2]. Left ventricular enlargement occurs when there is cardiac decompensation- the valve should be replaced prior to cardiac decompensation.
Before symptoms develop, survival is nearly normal even in
patients with severe stenosis [2]. Once symptoms develop prompt
surgery is required because survival decreases sharply (survival
without surgical intervention is 2-3 years at this point) [12]. In
the absence of valve replacement surgery 50% of patients with
angina die within 5 years, 50% of patients with syncope die within
3 years, and 50% of patients with congestive heart failure
symptoms die within 2 years [2]. For asymptomatic patients, a high
risk patient population can be identified by the flow velocity
across the valve [2]. When the peak transaortic velocity exceeds 4
m/sec (or a mean gradient of 64 mmHg) seventy percent of such
patients will become symptomatic within 2 years [2]. An aortic
gradient greater than or equal to 50 mm Hg should be considered
for intervention [1]. Patients with left ventricular systolic
dysfunction and a low transvalvular pressure have far advanced
disease and a poor prognosis (operative mortality 20%;
post-operative 4 year mortality 50%) [2]. Severe aortic wall
calcificaiton (porcelain aorta) is a clinically significant
preoperative finding that indicates aortic valve replacement
surgery may be technically difficult or impossible (aortotomy,
cross clamping, cannulation, and suturing are more likely to cause
embolic phenomena, dissection, or mural laceration) [12].
Valve replacements can be mechanical or bioprosthetic. Biologic
valves require no anticoagulation, but are prone to wear, whereas
mechanical valves are designed to last decades, but require
lifelong anticoagulation [14]. The prevalence of bioprosthetic
valve failure is about 30% for heterograft valves (made from
bovine tissue) and 10-20% for homograft valves (havasted from
cadavers) within 10-15 years and it is usually the result of
leaflet degeneration [13].
Complications following aortic valve surgery:
Paravalvular or valvular leak/regurgitation: Paravalvular
regurgitation occurs when blood flows abnormally through a channel
between the prosthesis and annulus as a result of incomplete
sealing [13,31]. It is a common complication of both mechanical
and bioprosthetic valves [31]. Paravavular leak/regurge can be
seen in 2-10% (up to 18%) of patients following aortic valve
replacement surgery and in 7-17% of patients after mitral valve
surgery [13,31]. The leak may be precipitated by dehiscenced
sutures, improper implantation/suturing of the valve to the
annulus, or endocarditis-induced dehiscence (this is the most
common cause) [13,31]. Patients may be asymptomatic or have mild
hemolytic anemia [13]. If not related to infection, minor
paravavular leaks are generally benign and well tolerated [13].
Large PVLs are seen in less than 1% of surgeries and can result in
heart failure, hemolytic anemia, or endocarditis [31]. If
treatment is necessary, surgical or transcatheter closure can be
performed [13,31]. At CT, the leak appears as a contrast-filled
channel adjacent to the valve area that is continuous with the LV
outflow tract and ascending aorta [13].
Valve dehiscence: Dehiscence- suture line breakdown leading to
separation of the valve from the annulus- is a serious
complication that requires prompt diagnosis and surgical
correction [13]. The most common cause is infective endocarditis,
but it can also occur as a result of an ascending aortic aneurysm
[13]. Dehiscence generally initially manifests as a paravalvular
leak [13]. Plain film findings suggestive of dehiscence are a
change in angulation of a valve by more than 6? (virtually
diagnostic) [13]. At CT, dehiscence appears as a gap between the
aortic annulus and the opposing margin of the artificial valve
with a continuous column of contrast from the LV into the aortic
root [13].
Prosthetic valve endocarditis and abscess formation: PHV
endocarditis has a prevalence of 1-6%, with a mortality rate as
high as 40% [31]. The risk for endocarditis is greatest during the
first 5 years following surgery [13]. It is classified as early or
late using a cutoff of two months following surgery [13]. Early
endocarditis is usually caused by perioperative contamination and
Staph epidermidis (30%) and Staph aureus (20%) are the most common
organisms [13,31]. Late endocarditis is most commonly associated
with Streptococci infection through hematogenous seeding, similar
to native valves [13,31]. For mechanical valves, the infection
often starts at the sewing cuff, whereas bioprosthetic valve
infection is similar to native endocarditis (typically involving
the valve cusps) [13].
A perivalvular abscess is an infected blood-containing space
adjacent to a PHV [31]. Extension of bacterial infection to the
perivalvular region is more common in PHVs (56-100%) than in
native valves (10-40%), especially in the aortic position-
typically into the mitral-aortic intervalvular fibrosis
(aortomitral curtain) [31]. Increased wall thickness of the aorta
(>5mm) is an early sign of aortic root infection and a
thcikened hypoattenuating area surrounding the aortic root is
suggestive of a paravalvular abscess [31].
Aortic dissection: Valve replacement is an independent risk
factor for type A aortic dissection (0.6% of cases post
operatively) [13].
Pseudoaneurysm formation: Pseudoaneurysm formation is a
complication associated with procedures involving replacement of
the aortic root or ascending aorta and can be seen in 7-25% of
patients with composite grafts [13]. Higher risk is seen in
patients with connective tissue disease, hypertension, infection,
and aortotomy blowout [31]. The pseudoaneurysm appears as a
contrast filled saccular outpouching [31].
Transcatheter aortic valve
implantation/replacement (TAVI or TAVR):
TAVI has been developed for treatment of high risk patients with
severe aortic stenosis [10]. The 30 day mortality from the
procedure is 7% and the mortality at one year is approximately 24%
[25]. The stroke rate is 4% [25]. Risk factors associated with an
increased mortality at one year include advanced age (95 years or
over), male sex, end-stage renal disease, severe COPD,
nontransfemoral access, STS PROM socre greater than 15%, and
preoperative atrial fibrillation [25].
Two systems are being used in the clinical setting- the
balloon-expandable Edwards SAPIEN with a stainless steel frame
(and SAPIEN XT with a cobalt-chromium frame and which is not
available in the US) and the self-expandable Medtronic CoreValve
ReValving System (also not available in the US) [19,24]. The
Sapien valves both have valve leaflets made of bovine pericardium
[24]. The CoreValve consists of a trileaflet procine valve mounted
on a self-expanding hourglass-shaped nitinol frame which is
markedly longer (53-55mm and extends beyond the sinotubular
junction into the ascending aorta) than the balloon expandable
SPAIEN valve [19]. For the SPAIEN valve, the annulus must measure
between 18-27 mm; for the CoreValve the size must range between
20-29 mm [19]. The Sapien valves remain within the aortic root due
to their shorter frames and do not extend above the sinotubular
junction [24]. Along the inferior border, neither valve should
extend beyond the level of the native annulus [24].
The transcatheter aortic valve prostheses are anchored in the
aortic annulus and displace the native aortic wall cusps toward
the aortic wall [15]. The valve is inserted via a large diameter
delivery sheath (over 18 French) and requires good vascular access
for deployment [10]. The preferred method of delivery is via the
femoral artery [15]. However, in the setting of unfavorable pelvic
vascular anatomy the device can be delivered via the subclavian
artery or LV apex using a minimally invasive thoracotomy (Sapien
valve only; the transapical route is not available for placement
of self-expanding stents) [14,15,19]. An aortic approach (entry
into the ascending aorta after mini-thoractomy using the second
intercostal space) is also possible [15]. Patients with an aortic
valve annulus that measures less than 18 mm are not candidates for
the procedure as there is no suitable valve available for these
patients [15]. Deployment of the valves is now most commonly
preceded by balloon aortic valvuloplasty to facilitate the passage
of the prosthesis through the stenotic native aortic valve [19].
The SAPIEN valves are expanded by a balloon during burst
ventricular pacing to minimize cardiac output and to prevent
migration of the valve during deployment [19].
The presence of prosthesis patient mismatch is a predictor of mortality following TAVR [20]. Mismatch can be seen up to 39% of cases [20]. Severe mismatch has been reported in 8-11% of patients with the balloon-expandable Edwards SAPIEN valves and 2-16% of patients receiving CoreValves (Medtronic) [20]. The goal of sizing for the prosthesis is to ensure that it is slightly larger than the aortic annulus (by 10-15% for balloon-expandable valves) in order to minimize post-procedure paravalvular regurge (there is evidence that oversizing is inversely related to paravalvular regurge [19]), but not so large as to result in vascular injury [17,19]. (Also- remember that a 10% over-sizing based on diameter actually represents a 21% over-sizing by area [21]. However, presently only certain sizes are available for balloon expandable prostheses (for annulus sizes between 18-29 mm) and therefore, the degree of oversizing may be greater than is desired [17,23]. CT imaging plays an important role in determining the appropriate sized valve for implantation [15,16,17]. CT has been shown to be superior to TEE for proper sizing of the valve prosthesis [17]. Because of the commonly non-circular configuration of the annulus, annular area by 3-dimensional MDCT provides a reproducible measure to determine proper valve sizing [20]. Compared to CT, TEE underestimates annulus dimensions by 1.5 mm =/- 2.3 [19]. TEE can lead to undersizing of the valve prosthesis in 33% of patients and this can be associated with an increased risk for paravalvular regurge [17]. TEE can also lead to oversizing by ≥ in 10% of patients and this can be associated with a higher risk of annular injury [17]. Another benefit of CT is that it can be used to preoperatively determine a suitable fluoroscopic projection for the actual valve implantation procedure (during fluoroscopic implantation of the device it is important to achieve an exactly orthogonal view through the aortic valve plane) [18].
The aortic root extends from the LVOT to the sinotubular junction
which marks the transition from the aortic sinuses to the tubular
ascending aorta [23]. CT imaging of the aortic root should be
performed with either retrospective gating or prospective ECG
triggering and 1 mm thick slices [15]. Beta blockers are not
administered for the exam as they may depress LV systolic function
and thereby worsen symptoms caused by critical aortic stenosis
[19]. The root is widest at the midpoints of the sinus and
narrowest at the basal attachment of the leaflets and the
sinotubular junction [19]. The remainder of the aorta, iliac, and
common femoral vessels should also be imaged because vascular
complications associated with the procedure are usually related to
significant atherosclerosis (due to stroke caused by dislodged
plaque), severe vascular calcification, dissection, an external
sheath diameter that exceeds the minimal arterial diameter, and
vascular tortuosity and kinking [15,19]. CT data sets should be
evaluated to the presence of LV thrombi [15].
|
|
|
The aortic annulus is not a real anatomic structure [23]. The
aortic annulus is formed by the three lowest points of the aortic
valve cusps where they connect to the wall of the LVOT [15]. The
aortic annulus has been shown to be of a non-circular, often oval
shape [19]. The aortic root is a dynamic structure, with the
aortic annulus not only subject to pulsatile changes, but also
contour deformity related to movement of the aorto-mitral junction
or changing volume and pressure in the left atrium [19]. Accurate
measurement of the aortic annulus is critical to choosing the
appropriate prosthesis size [15]. If the prosthesis is too small,
paravalvular regurge can occur, or even embolization of the
prosthesis [15]. If the prosthesis is too large relative to the
annulus, rupture may occur (which is often fatal) [15]. For
accurate measurement, the CT data set much be manipulated to
create an image that exactly corresponds to the basal ring of the
aortic valve (annulus). For measurement, systolic images are
preferable over diastole because the annulus and aortic root are
slightly larger during systole (mid-systole corresponds to 10-30%
of the R-R interval) and diastolic images can result in
undersizing of the prosthesis (the dimension of the aortic annulus
can vary up to 5 mm between systole and diastole [23])
[15,16,17,19,28]. The annulus has an ovoid, non-circular shape,
but it will reshape to a more circular geometry following
implantation of the prosthesis [15]. Three measurements should be
performed: 1- the long and short diameters (DL and DS)
and from these a mean diameter should be determined; 2- the
planimetry area of the aortic annulus and the area of the
calcification; and 3- the circumference of the aortic annulus
[15].
It is also important to include the distance of the coronary
ostia to the aortic valve plane, aortic cusp width, width of the
aortic sinus, width of the sinotubular junction, and width of the
ascending aorta [15]. These measurements are important because the
native leaflets and calcifications will be displaced/crushed by
the prosthesis with a risk of potential coronary occlusion-
particularly with shallow sinuses and heavily calcified cusps
[15,19]. Occlusion of the coronary ostia can occur in 0.6-4.1% of
cases [23]. A minimum distance of 10-14 mm between the coronary
ostia and the leaflet insertion are suggested- however, the length
of the aortic valve cusps and extent of calcification need to be
taken into consideration (ie: the distance should be greater than
the length of the leaflet) [15,23]. Other features that may be
predictive of coronary occlusion include shallow sinuses of
valsalva (width of the sinuses of Valsalva should be ≥ 30mm) ,
long aortic valve cusps, and a narrow sinotubular junction [15].
Aortic valve calcification has also been speculated to be
associated with an increased risk for prosthesis dislodgement
[15].
If the valve is deployed too low, there is an increased risk of
heart block, paravalvular regurgitation, and mitral valve
dysfunction [19]. If the valve is deployed to high, there is
increased risk of valve embolization into the aorta, paravalvular
regurgitation, and aortic root injury [19].
Paravalvular regurgitation: Reguritant leakage of blood around
the attachement sites of the prosthetic valve is a relatively
common complication with important clinical consequences [23]. PVR
(even mild) is associated with an increased risk for short and
long-term mortality [22]. Moderate to severe paravalvular aortic
regurgitation (PVR) can occur in up to 11% of patients with
echocardiographic sizing of the prosthesis [7]. Factors associated
with an increased risk for PVR include: 1- a size mismatch between
the chosen valve and the annular dimensions; 2- asymmetric and/or
incomplete deployment of the valve due to interposition of native
aortic valve calcifications between the valve and the aortic root
wall; and 3- incorrect positioning of the valve in the aortic root
[24]. The risk for PAR is significantly higher for undersized
compared to oversized prostheses (20% vs 7%) [17,23].
Para-valvular regurge has shown an association with increased
in-hospital and mid-term mortality [19]. Another risk factor for
paravalvular regurge is the presence of severe/excessive aortic
valve calcification which can hamper the apposition of the
prosthesis to the aortic root/annulus- resulting in gaps between
the prosthetic frame and the native aorta which can lead to
paravalvular regurge (PVR) [15,19,22,23]. Dense leafleft
calcification with an Agaston score of greater than 3000 or a mass
of greater than 800mg/mm2 are markers for immediate
post deployment paravalvular regurge [19]. Large nodular
calcifications on the valve are also associated with an increased
risk for PVR [22].
Aortic root injury: There is an increased risk for aortic root injury in balloon-expandable TAVR associated with more than 20% over-sizing and with the presence of left ventricular outflow calcification- especially when the calcification is located below the non-coronary cusp and extending from the annular region [27]. Self-expanding stents have been shown to have a relatively higher incidence of sub-annular injury as manifested by atrioventricular block [17].
Mortality in the 30 days following the transarterial procedure is
between 4-12% [11]. Stroke can complicate the procedure in 0-9%
(6.7% [24]) of patients [11]. Iliofemoral dissection or
perforation can occur in 8% of patients [11]. Moderate-to-severe
arterial calcification is associated with a 3-fold increase in
vascular complications (29% versus 9%) and special caution is
indicated if the calcification is circumferential, nearly
circumferential, and/or located at vessel bifurcations [15]. The
presence of a minimal arterial lumen diameter less than that of
the external sheath is associated with a 4-fold increased (23% vs
5%) [15]. A sheath-to-femoral artery ratio of ≥ 1.05 is
predictive of vascular-access related complications and 30-day
mortality [15]. Survival at one year has been reported to be 74%
[11].
Acute kidney injury: AKI can be seen in 12-34% of patients
following TAVR and can be associated with increased post-operative
morbidity and mortality [30]. AKI is more likely to develop in
patients with bilateral renal artery stenosis greater than or
equal to 50%, and in those with severe atherosclerotic
calcification of the aorta and iliac arteries on CT [30].
X-ray:
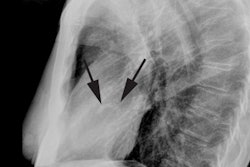
CXR: On CXR, nearly half of patients with aortic stenosis will have a normal film. Early concentric LV hypertrophy (due to the pressure overload) may be characterized by rounding of the left ventricular contour (a "left ventricular" configuration to the heart). There is frequently post stenotic dilatation of the ascending aorta and a prominent ascending aorta in a patient under the age of 30 years is unusual and should be further evaluated. The degree of aortic enlargement bears no relationship to the severity of stenosis. Valvular calcification can be seen (best appreciated on the lateral view) and a calcific valve generally indicates a gradient of 50 mm Hg across the valve (i.e.: a significant stenosis). The pulmonary vascularity is normal. Later the presence of cardiomegaly and pulmonary venous hypertension indicate decompensation.
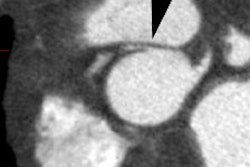
CT: Aortic valve calcification is a common finding on CT and is
usually clinically not significant. Aortic stenosis should be
suspected, however, if calcification is seen in patients under the
age of 55 years or if the calcification is moderately dense. In
general, the more severe the calcification, the more significant
the stenosis and gradient across the valve [3,6]. Aortic valve
calcium has also been shown to be an indpendent predictor of
increased all-cause mortality [16].
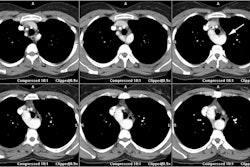
MDCT permits accurate non-invasive assessment of the aortic valve area and has excellent sensitivity and specificity to detect severe stenosis [6,7,9]. Optimal image quality for aortic valve planimetry is best achieved during midsystole (approximately 20% of the R-R interval or 50-100 msec from the R-wave peak depending on heart rate) [8]. A limited scan range can be used and the amount of contrast can also be decreased to as low as 35 mL [9]. Compared to TTE, the aortic valve area tends to be overestimated by CT- however, due to mathematical models used for valve area determination on TTE, the CT measurement is actually more accurate [9].
MRI: On MRI a bicuspid aortic valve can be recognized by its thickened valve leaflets. The leaflets are characteristically "dome" into the aorta. Ascending aortic dilatation and LV hypertrophy are also readily apparent and, in long standing cases, slightly increased, heterogeneous signal may be identified within the LV myocardium. Cine gradient echo images will demonstrate the thickened, poorly mobile aortic valves and a signal void of turbulent flow across the aortic valve during systole.
REFERENCES:
(1) Radiologic Clinics of North America 1999; Lipton MJ, Coulden
R. Valvular heart disease. 37(2): 319-339
(2) J Nucl Cardiol 2002; Carabello B. Pathophysiology of valvular heart disease: implications for nuclear imaging. 9: 104-113
(3) AJR 2006; Liu F, et al. Aortic valve calcification as an incidental finding at CT of the elderly: severity and location as predictors of aortic stenosis. 186: 342-349
(4) AJR 2006; Gilkeson RC, et al. MDCT evaluation of aortic valvular disease. 186: 350-360
(5) Radiology 2006; Alkadhi H, et al. Aortic stenosis: comparative evaluaiton of 16-detector row CT and echocardiography. 240: 46-55
(6) Radiology 2007; Pouleur AC, et al. Aortic valve area assessment: multidetector CT compared with cine MR imaging and transthoracic and transesophageal echocardiography. 244: 745-754
(7) AJR 2008; La Bounty TM, et al. Aortic valve area on 64-MDCT correlates with transesophageal echocardiography in aortic stenosis. 191: 1652-1658
(8) Radiographics 2009; Chen JJ, et al. CT angiography of the cardiac valves: normal, diseased, and postoperative appearances. 29: 1393-1412
(9) J Cardiovasc Comput Tomogr 2011; Pflederer T, Achenbach S.
Aortic valve stenosis: CT contributions to diagnosis and therapy.
4: 355-364
(10) J Cardiovasc Comput Tomogr 2011;
Schoenhagen P, et al. Computed tomography evaluation for
transcatheter aortic valve implantation (TAVI): imaging of the
aortic root and iliac arteries. 5: 293-300
(11) Circulation 2009; Webb JG, et al.
Transcatheter aortic valve implantation. Impact on clinical and
valve related outcomes. 119: 3009-3016
(12) Radiographics 2012; Bennett CJ,
et al. CT and MR imaging of the aortic valve:
radiologic-pathologic correlation. 32: 1399-1420
(13) Radiographics 2012; Pham N, et al. Complications of aortic
valve surgery: manifestations at CT and MR imaging. 32: 1873-1892
(14) Radiographics 2012; Habets J, et al. Multidetector CT
angiography in the evaluation of prosthetic heart valve
dysfunction. 32: 1893-1905
(15) J Cardiovasc Comput Tomogr 2012; Achenbach S, et al. SCCT
expert consensus document on computed tomography imaging before
transcatheter aortic valve implantation (TAVI)/transcatheter
aortic valve replacement (TAVR). 6: 366-380
(16) J Cardiovasc Comput Tomogr 2012; Buttan Ak, et al.
Evaluation of valvular heart disease by cardiac computed
tomography assessment. 6: 381-392
(17) J Cardiovasc Comput Tomogr 2012; Willson AB, et al. Computed
tomography-based sizing recommendations for transcatheter aortic
valve replacement with balloon-expandable valves: comparison with
transesophageal echocardiography and rationale for implementation
in a prospective trial. 6: 406-414
(18) J Cardiovasc Comput Tomogr 2012; Arnold M, et al. A method
to determine suitable fluoroscopic projections for transcatheter
aortic valve implantation by computed tomography. 6: 422-428
(19) Radiology 2013; Blanke P, et al. CT in transcatheter aortic
valve replacement. 269: 650-669
(20) J Cardiovasc Comput Tomogr 2013; Freeman M, et al.
Multidetector CT predictors of prosthesis-patient mismatch in
transcatheter aortic valve replacement. 7: 248-255
(21) J Cardiovasc Comput Tomogr
2014; Blanke P, et al. Oversizing in transcatheter aortic valve
replacement, a common used term but a poorly understood one:
dependency on definition and geometrical measurements. 8: 67-76
(22) J Cardiovasc Comput Tomogr 2014; Azzalini L, et al. The
aortic valve calcium nodule score (AVCNS) independently predicts
paravalvular regurgitation after transcatheter aortic valve
replacement. 8: 131-140
(23) Radiographics 2014; Salgado RA,
et al. Preprocedural CT evaluation of transcatheter aortic valve
replacement: what the radiologist needs to know. 34: 1491-1514
(24) Radiographics 2014; Salgado RA,
et al. Transcatheter aortic valve replacement: postoperative CT
findings of Sapien and CoreValve transcatheter heart valves. 34:
1517-1536
(25) JAMA 2015; Holmes DR, et al.
Clinical outcomes at 1 year following transcatheter aortic valve
replacement. 313: 1019-1028
(26) Radiographics 2015; Sheth PJ, et
al. Cardiac physiology for radiologists: review of relevant
physiology for interpretation of cardiac MR imaging and CT. 35:
1335-1351
(27) J Cardiovasc Comput Tomogr 2015; Hansson NC, et al. The
impact of calcium volume and distribution in aortic root injury
related to balloon-expandable transcatheter aortic valve
replacement. 9: 382-392
(28) J Cardiovasc Comput
Tomogr 2016; Murphy DT, et al. Dynamism of the aortic annulus:
effect of diastolic versus systolic CT annular measurements on
device selection in transcatheter aortic valve replacement (TAVR).
10: 37-43
(29) J Nucl Cardiol 2017; Hussalin N, et al. An assessment of the
safety, hemodynamic response, and diagnostic accuracy of commonly
used vasodilator stressors in patients with severe aortic
stenosis. 24: 1200-1213
(30) AJR 2018; Kandathil A, et al. Atherosclerosis on CT
angiogram predicts acute kidney injury after transcatheter aortic
valve replacement. 211: 677-683
(31) Radiographics 2019; Rajiah P, et al. Multimodality imaging
of complications of cardiac valve surgeries. 39: 932-956