Mitral Stenosis:
Clinical:
The mitral apparatus consists of an annulus, two valve leaflets (anterior and posterior- each of which consists of three scallops), chordea tendoneae, and papillary muscles [6,7]. The mitral valve annulus is a D-shaped ring within the left atrioventricular groove and is the site of the valve leaflet attachement - its bordered by the lef circumflex artery and the coronary sinus [6]. The straight border of the "D" is formed by the anterior portion of the annulus, which is in fibroous continuity with the aortic valve [6]. The curved border of the D is formed by the posterior annulus which is attached to the pliant endocardium [6]. Because the mitral annulus is D-shaped, the two mitral leaflets, anterior and posterior, are not equal in size [6]. The anterior leaflet attaches to roughly one-third of the annulus, but covers more of the valve orifice than the narrower posterior leaflet [6]. The anterior leaflet also forms part of the LV outflow tract [6]. Normal leaflet thickness is less than 5 mm [6]. The mitral valve leaflets are supported by chordae, which are attached to the papillary muscles [6]. The posteromedial muscle has a single blood supply and is more vulnerable to ischemia and rupture compared to the anterolateral muscle [6]. LV function, especially the lateral wall where the papillary muscles arise, has an important effect on mitral valve function [6]. Wall motion abnormalities, ventricular dilatation, or both can alter tensile forces on the chordae and result in valve dysfunction [6].The normal area of the mitral valve is 4 to 6 cm2 [5].
Increased resistance to antegrade flow with an increasing
transvalvular
pressure gradient occurs as the mitral valve area falls below 2.5
cm2
[5]. A valve area of greater than 1.5 cm2 is
mild stenosis and between 1.0- 1.5 cm2 is moderate
stenosis [8]. Severe stenosis is associated
with areas less than 0.8-1.0 cm2, and most valves are
replaced
when their area
becomes less than 1.1 cm2. Mean valve pressure
gradients of less than 5 mm Hg are mild stenosis, between 5-10 mm
Hg are moderate, and greater than 10 mm Hg is severe stenosis [8].
Mitral stenosis results in increased resistance to emptying of the left atrium. This produces a reduced left ventricular output and an increase in pulmonary venous pressure (a valveless system, thus LA pressures are transmitted directly to the vessels). Eventually this increased pressure is back transmitted to the pulmonary arteries. The pulmonary arteries undergo medial hypertrophy and intimal sclerosis in response, and pulmonary arterial hypertension results. Ultimately, the right ventricle hypertrophies, dilates, and then fails. Clinically there is a diastolic murmur and resting tachycardia as the heart attempts to supply more blood systemically. Rarely, patients with mitral stenosis can present with diffuse alveolar hemorrhage [2]. Another rare, late sequella of mitral stenosis is parenchymal ossification [2]. In approximately 0.6% of cases of mitral stenosis, a coexisting ASD may relieve the left atrial hypertension and promote the formation of a left to right shunt. This combination of findings is known as Lutembacher syndrome [2].
The two major factors influencing prognosis in mitral stenosis are the presence of pulmonary hypertension (triples operative mortality) and the presence of symptoms [3]. Once more than mild symptoms develop, the prognosis for medical treatment decreases [3].
Etiologies of mitral stenosis include rheumatic heart disease (most commonly), congenital mitral stenosis, or an obstructing lesion such as a left atrial myxoma.
X-ray:
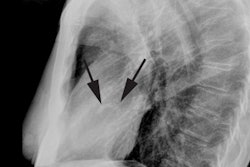
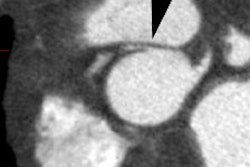
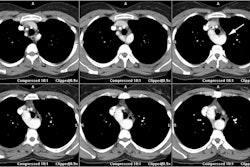
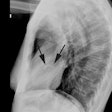
CXR: The left atrial appendage is the only portion of the left atrium that forms part of the left border of the heart. On PA radiographs it occupies the portion of the left heart border between the main pulmonary artery segment and the superior portion of the left ventricular contour. When left atrial pressure and volume are normal, this segment of the left heart border is concave. Early or mild enlargement of the left atrium may be detected as enlargement of the left atrial appendage with straightening of this segment of the left heart border. With continued enlargement, this segment will become convex. Another finding of left atrial enlargement include a "double density" in the mid-portion of the cardiac silhouette on the frontal view. A line from the mid-point of the right border of the double density to the midpoint of the border of the left mainstem bronchus should measure less than 7.5 cm (7.0 cm in females). A normal left atrium should also lie anterior to a line drawn down the center of the trachea on the lateral, non-rotated view. Other findings which can suggest LA enlargement include posterior esophageal displacement on barium swallow, elevation of the left mainstem bronchus, and straightening of the left heart border due to enlargement of the left atrial appendage. In cases of long-standing stenosis, the LA wall may calcify. Mitral valve calcification is only seen 10% of cases (Note: Calcification of the mitral valve annulus does not indicate mitral stenosis). Pulmonary venous congestion can be seen as the stenosis progresses. Since the left ventricle is unaffected by mitral stenosis it will remain normal [2]. Later there is pulmonary arterial hypertension and right ventricular enlargement.MDCT: Mitral valve leaflet calcification indicates mitral valve sclerosis or stenosis [4]. For most patients, 65% of the R-R interval is the best phase for multiplanar reformatted imaging of the open mitral valve, and 5% of the R-R interval is the best phase for imaging of the closed mitral valve [5]. Restricted opening of the thickened valve produces a "fish-mouth" appearance on short axis images [6]. Bowing of the thickened and fibrotic anterior leaflet produces a "hockey-stick" appearance that is best seen on two or four-chamber view images [6].
MRI: On MRI other findings of mitral stenosis include a mild increased signal intensity in the lungs on spin echo images due to pulmonary venous hypertension and interstitial edema. Cine gradient images can be used to demonstrate turbulent flow across the mitral valve which appears as a fan-shaped signal void in the LV below the valve during diastole.
REFERENCES:
(1) J Thorac Imag 1994; Boxt LM, et al. Normal plain film examination of the heart and great arteries in the adult. 9: 208-218
(2) Radiographics 1999; Woolley K, Stark P. Pulmonary parenchymal manifestations of mitral valve disease. 19: 965-972
(3) J Nucl Cardiol 2002; Carabello B. Pathophysiology of valvular heart disease: implications for nuclear imaging. 9: 104-113
(4) AJR 2007; Mahnken AH, et al. MDCT detection of mitral valve calcifcation: prevalence and clinical relevance compared with echocardiography. 188: 1264-1269
(5) Radiographics 2009; Chen JJ, et al. CT angiography of the cardiac valves: normal, diseased, and postoperative appearances. 29: 1393-1412
(6) Radiographics 2010; Morris MF, et al. CT and MR imaging of the
mitral valve: radiologic-pathologic correlation. 30: 1603-1620
(7) J Cardiovasc Comput Tomogr 2012; Buttan Ak, et al. Evaluation
of
valvular heart disease by cardiac computed tomography assessment.
6:
381-392
(8) Radiographics 2015; Sheth PJ, et al. Cardiac physiology for radiologists: review of relevant physiology for interpretation of cardiac MR imaging and CT. 35: 1335-1351