[In-111-DTPA-D-Phe-] Octreotide: (Pentetreotide In-111) Tumor Imaging
Somatostatin is a small peptide hormone of 14 amino acids produced in the hypothalamus and pancreas. It's hormonal actions include inhibition of the release of growth hormone, insulin, glucagon, and gastrin. Somatostatin receptors are integral membrane glycoproteins that are distributed in a variety of tissues throughout the body. Somatostatin receptors are found on many cells of neuroendocrine origin, such as the somatotroph cells of the anterior pituitary and the pancreatic islet cells. Cells not classically regarded as neuroendocrine, such as lymphocytes, may also have these receptors. Receptors have also been identified on many endocrine related tumors such as Apudomas. These neoplasms have the ability to synthesize amines and polypeptides by virtue of amino precursor uptake and decarboxylation activity. Types of Apudomas include: Pancreatic endocrine (islet cell) tumors (gastrinoma, insulinoma, glucagonoma, VIPoma), carcinoid tumors, small cell lung carcinomas, neuroblastomas, pheochromocytomas, paragangliomas, medullary thyroid carcinoma, and certain pituitary tumors. Other tumors can also have somatostatin receptors such as: Hodgkins and non-Hodgkins lymphoma, certain brain tumors- meningiomas and astrocytomas, breast cancer, Merkel cell tumors of the skin, and granulomatous disease (Sarcoid). Other granulomatous diseases such as tuberculosis, Wegener's granulomatosis, DeQuervain's thyroiditis, and aspergillosis have also been shown to contain somatostatin receptors in vivo [1].
Octreotide is a somatostatin analogue consisting of eight amino acids. In-111 is chelated to octreotide using DTPA and the resulting radiopharmaceutical is called In-111 pentetreotide (Octreoscan) [31]. The agent is longer acting than the native hormone in vivo because of resistance to enzyme attack (half-life of 2 to 3 hours as opposed to 2-3 minutes for the native hormone). There are 6 somatostatin receptor subtypes - 1, 2A, 2B, 3, 4 , and 5 (all of which bind somatostatin), but only subtypes 2 and 5 bind pentetreotide [31,50]. Pentetreotide has been shown to bind to somatostatin receptors on both tumor and non-tumor sites throughout the body. Because 80% of enteropancreatic neuroendocrine tumors express subtype 2 receptors, a majority of abdominal neuroendocrine tumors are Octreoscan positive [31]. After internalization through the somatostatin receptor system the agent is degraded to the final radiolabeled metabolite 111In-DTPA-D-Phe in the lysosomes [8]. This metabolite is not capable of passing through the lysosomal or other cell membrane and will remain within the cell [8]. The agent is then translocated to the perinuclear area and into the nucleus [7]. Pentetreotide has been shown to detect 84% to 91% [26,39] of neuroendocrine tumors and is superior to MIBG for the detection of gastroenteropancreatic neuroendocrine tumors [26].
Auger electrons emitted from the In-111 (although low energy [0.5-25keV] and short ranged) can produce cytotoxic effects due to their proximity to the nucleus [7]. Clinical trials have been performed evaluating the use of high doses of In-111-DTPA Octreotide (150-200 mCi) for possible therapeutic applications in treating neuroendocrine neoplasms [8]. Other agents such as the high-energy beta-emitter Y-90 may also hold promise for therapeutic actions when labeled to Octreotide [8].
Because neuroendocrine tumors also demonstrate increased monoamine metabolism they can also be visualized with 123I-MIBG [39]. Unfortunately, overexpression of the noradrenalin transporter (which mediates uptake of MIBG) is found in only about 50% of NET's and apart from pheochromocytomas (in which MIBG has excellent sensitivity) MIBG imaging is less sensitivity than Octreotide for imaging NET's (52% versus 89%) [39].
Within an hour after injection most of the In-111 pentetreotide distributes from the plasma to the extravascular body tissues and by 20 hours only 1% of the dose remains in the blood pool. Tissue uptake is either related to the receptor status of target tissues or to the elimination of the radioligand. The normal pituitary gland (faint uptake), thyroid (faint uptake), liver, gallbladder, spleen, kidneys, and urinary bladder are visualized in most patients, as is the bowel to a variable degree. Faint diffuse breast uptake can also be seen in about 15% of female patients [8]. Normal uptake in the thyroid and liver may serve to mask lesions in these locations, but the kidenys and the spleen are the hottest organs on images. The spleen is the critical organ (14.5 rads/ 6 mCi). Octreotide is excreted almost exclusively by the kidneys (via glomerular filtration) with 85-90% of the dose recovered in the urine in the first 24 hours [17]. Uptake in the kidneys is for the most part from reabsorption of the radiolabeled peptide in the proximal renal tubular cells via megalin receptors after glomerular filtration [27]. Renal uptake can be reduced by the use of succinylated gelatin and this can have important implications for therapeutic uses of somatostatin receptor agents [27]. Somatostatin receptors have also been demonstrated in human renal tubular cells and the vasa recta [8]. There is little hepatobiliary excretion (2-10%), but bowel activity may sometimes be visualized on delayed 24 hour images, and a bowel prep should be given prior to imaging. Patients should be well hydrated during the exam to enhance renal clearance. The use of this agent in patients with impaired renal function should be carefully considered. The sensitivity of In-111 Pentetreotide may be reduced in patients concurrently receiving therapeutic doses of octreotide acetate (Sandostatin) for control of symptoms from certain tumors, although this has not been identified in clinical trials. It is nonetheless recommended that octreotide therapy be withheld for at least 24 to 72 hours prior to the exam if possible [2,17,31]. Octreotide in therapeutic doses has also been shown to produce severe hypoglycemia in patients with insulinomas. Although the agent is not expected to exert clinically significant effects, as pentetreotide is an octreotid analog, it could potentially also produce hypoglycemia. Patients suspected of having an insulinoma should have an I.V. solution containing glucose administered before and during the administration of the radiopharmaceutical. Transient adverse effects to Octreotide occur in less than 1% of patients and include dizziness, fever, flushing, HA, and hypotension.
Non-pathologic accumulation of the tracer has been demonstrated in the nasal and hilar regions of patients with URI's, and in the lung following external radiation or bleomycin chemotherapy. Thyroid uptake in patients with Graves' disease is increased and may be related to the presence of activated lymphocytes. Orbital uptake can be seen in patients with Graves' ophthalmopathy. Joint uptake can be seen in patients with rheumatoid arthritis associated with active inflammation and may be related to the presence of somatostatin receptor containing inflammatory cells [1]. In sarcoid nodal and parenchymal lung uptake are frequently seen [8]. Other sites of tracer accumulation include accessory spleens, recent cerebral vascular accidents, and recent surgical incisions [8].
Imaging has been successful in most patients with neuroendocrine tumors [24], and in patients with lymphomas, granulomatous diseases, or diseases in which activated lymphocytes play a role (such as Grave's ophthalmopathy [3]). In-111 Pentetreotide scan results have been shown to be consistent with the final diagnosis in 86% of patients. Compared with carcinoids (96% detection) and gastrinomas, lower success rates were noted for localization of insulinomas, neuroblastomas, pituitary adenomas, and medullary thyroid carcinoma. Specificity was approximately 50%. Imaging resulted in a change in patient management in 30% of cases. The sensitivity for the detection of liver metastases in patients with gastroenteropancreatic neuroendocrine tumors has been reported to be as high as 92% [24]. Dedifferentiation of certain tumors (such as carcinoids, neuroblastoma, and medullary thyroid carcinoma) results in a loss of somatostatin receptors that can result in falsely negative exams [26]. Symptomatic patients with positive scans can be treated with Octreotide with a high likelihood of control of the hormonal hypersecretion by the tumor.
False positive exams have occurred in the nasal and pulmonary hilar regions of patients with URI's; at sites of locally irradiated lung; and at sites of recent surgery. Bleomycin has been associated with increased pulmonary activity.
10 ug of the agent is labeled to 5-6 mCi of In-111 and two sets of images are obtained. SPECT imaging increases the sensitivity of the exam and gives better anatomic delineation compared to planar views [8]. The agent must be used within 6 hours of preparation. Planar and SPECT images are obtained at 4 hours (over the area of interest) and at 18-24 hours (from the head to the mid-thigh level) following injection. Early imaging is performed to avoid physiologic biliary radiotracer excretion that could potentially obscure lesion visualization [31]. The exam is positive in 80 to 90% of cases by 4 hours. Planar images are obtained with a double-head or large field of view gamma camera, equipped with medium-energy, parallel hole collimators. The pulse height analyzer windows are centered over both In-111 photopeaks (172 keV and 245 keV) with a window width of 20%. The acquisition parameters for planar images are 300,000 preset counts or 15 minute view of the head and neck and 500,000 counts or 15 minutes for the remainder of the body. Generally 5 to 10 minute images are required for the images. A whole body exam may be performed using a scan speed of 3 cm/min to 6.77 cm/min [31]. Using higher scan speeds, such as 8 cm/min, will result in failure to recognize small lesions or lesions with a low density of somatostatin receptors [8]. SPECT images with a dual headed camera are acquired with a 180 degree rotation using a 128 x 128 matrix and a Hanning-Nyquist filter [31]. For a ttriple headed camera the acquisition parameters are: 40 steps or 3 degrees each and at least 30 seconds per step (45 seconds for brain SPECT). Delayed images at 48 hours may also be obtained if necessary- particularly if there is a large amount of bowel activity on 24 hour images (a laxative should also be used to help clear bowel activity) [8].
Specific Tumors:
About 75% of stage I and stage II breast cancers (lesions under 2 cm and between 2 to 5 cm, respectively) were detected, as were non-palpable axillary nodes in 4 of 13 patients. Patients with positive exams tended to have better 5 year survival rates (82 vs. 46%) [1].
Somatostatin receptors are found on 50-75% of small cell lung cancers [4]. Between 63-100% of the primary tumors can be detected on Octreotide scintigraphy, but the degree of uptake is variable and not dependent on lesion size [1,4]. Detection of metastatic disease to regional nodes and distant sites is variable (sensitivity 45-80%) [4].
Although the detection of primary non-small cell tumors in one study [1] was also high, metastatic lesions were not identified. Since somatostatin receptors are absent on most non-small cell lung cancers, uptake within these lesions may be related to receptor positive immune cells surrounding the tumor [1].
Carcinoid tumors secrete excessive amounts of vasoactive substances and neuropeptides including serotonin, histamine, bradykinin, substance P, and neurokinin-A [31]. Carcinoid tumors are relatively slow growing, however, about 40% of patients have metastases at the time of presentation [31]. Most carcinoid tumors arise from the GI tract (73-85% of cases) [31]. Carcinoid tumors may be found anywhere in the GI tract, however, the appendix is the most common location (75% of cases and almost invariably benign). The ileum is the most common location outside the appendix (90% of cases). For tumors not located in the appendix, metastases are observed in less than 2% of cases in which the tumor is less than 1 cm in size, but are detected in about 90% of cases once the lesion exceeds 2 cm. Other sites for carcinoid tumors include the bronchopulmonary system (10-29% of cases) and rarely other organs such as the kidney, thymus, larynx, and ovary [31].
Serotonin is the major metabolic product of most carcinoid tumors [31]. Serotonin is degraded to 5-hydroxyindoleacetic acid (HIAA) which is excreted in the urine and urinary 5-HIAA levels can be used to confirm the diagnosis of a carcinoid tumor [31]. Serotonin hypersecretion by carcinoid which has metastasized to the liver produces the characteristic "carcinoid syndrome" which includes flushing, diarrhea, bronchoconstriction, and right sided valvular heart disease. It may also occur in patients with bronchial, ovarian, or retroperitoneal tumors.
Between 80-100% of carcinoid tumors contain somoatostatin receptors [31]. Octreotide has an overall sensitivity of about 86% in the detection of carcinoid tumors (range 71-100%) (compared to 55-70% sensitivity for MIBG imaging[31]) [18]. In one study, in a small number of patients (n=33), Octreotide accumulation was identified in sites which were previously unsuspected or not recognized with other imaging modalities in 23 of 33 patients and detected at least one lesion in 15% of patients in whom no abnormalities were found relying on conventional imaging alone [5]. Unfortunately, confirmation that these abnormalities represented tumor was obtained in only eight patients and confirmation was not necessarily made by histologic analysis. Small lesions (under 1 cm) are frequently not identified on Octreotide imaging [18].
|
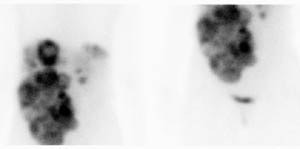
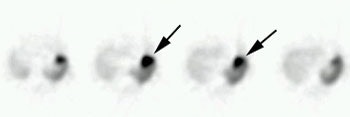
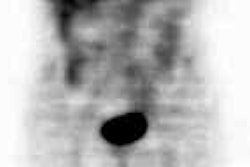
Carcinoid: The patient shown below had metastatic carcinoid to the liver. The planar Octreotide images demonstrate multiple areas of abnormal tracer uptake within the liver which is massively enlarged. (Click planar image to view axial SPECT images). |
|
|
Paragangliomas are very rare tumors of neural crest origin that most commonly arise from parasympathetic ganglia and rarely from sympathetic ganglia. Pheochromocytomas are paragangliomas that arise in the adrenal gland [45]. The most common location for extra-adrenal paragangliomas is the head and neck. These tumors have also been referred to as chemodectomas which arise from the chemoreceptor tissue of the carotid body, glomus jugular, or aortic body. Paragangliomas are referred to by their location- 1) carotid body (at the carotid bifurcation- this is the most common location [29]); 2) jugulare (in the jugular fossa along the tympanic branch [Jacobson's nerve] of the glossopharyngeal nerve and the auricular branch [Arnold's nerve] of the vagus nerve [29]); 3) vagale (along the course of the vagus nerve- most commonly at the ganglion nodosum (inferior ganglion) of the vagus nerve [29]; and 4) tympanicum (in the middle ear along Jacobson's nerve near the cochlear promontory [29]). These tumors can over-express somatostatin receptors and can be detected by Octreotide [25]. SST2 is the most prevalent receptor expressed in paragangliomas and SST1 is also strongly expressed in some tumors [45]. There is low expression of SST5 - a major difference from some endocrine tumors of the GI tract [45]. Paragangliomas can be multicentric in up to 10% of cases [25], but this can be as high as 32% in familial cases.
About 9-25% of paragangliomas are familial/hereditary- the
associated gene (postulated to be on chromosome 11) is transmitted
with an autosomal dominant mode of inheritance with incomplete
penetrance [20,45]. Children of male gene carriers have a 50%
incidence of tumor, whereas children of female gene carriers never
express the tumor [20]. Other susceptibility genes for
paragangliomas include succinate dehydrogenase subunit (SDH) B, C,
or D germline mutations, von Hipple Lindau disease, multiple
endocrine neoplasia type 2, neurofibromatosis type 1, and
suscpetability genes SDHA, SDHAF2, transmembrane protein 127, and
myc-associated factor X [45]. Patients with succinate
dehydrogenase subunit B, C, or D germline mutations are
particularly at risk for multiple lesions [33]. Malignant behavior
occurs in about 10% of cases (vagal lesions have a higher
incidence of malignancy (10-19%) [25]. Paragangliomas with an
underlying SDHB mutation are associated with aggressive behavior
and the development of metastatic disease in about 30% of carriers
[45]. In patients who have had surgery for paraganglioma,
recurrence or residual tumor is observed in 10% of carotid body
paragangliomas and in 29-50% of jugulotympanic paragangliomas
[19].
With octreotide imaging, paragangliomas are correctly identified in 94-95% of patients. Lesions as small as 1 cm can be detected [20]. **Unsuspected sites of disease where discovered in 30% to 36% of these patients through the addition of Octreotide imaging. Octreotide can also be used to screen patients with suspected familial paragangliomas for the detection of clinically occult lesions [20]. Octreotide imaging can also be sued to detect local recurrence or residual tumor following surgery which may occur in 15-30% of patients [25]. Octreotide imaging is superior to MIBG in the imaging of paragangliomas. [1,5,19,33] In one article focused on the detection of head and neck paragangliomas octreotide had a lesion based sensitivity of 89% versus 42% for MIBG [33].
|
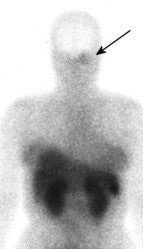
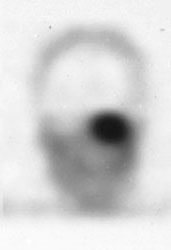
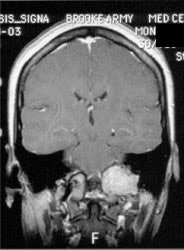
Glomus tumor: The octreotide exam was performed to assess for multifocality or metatstatic disease in this patient with a very large glomus tumor. Although the primary lesion is evident on whole body imaging (black arrow), SPECT images demonstrate the lesion more clearly (center). A coronal MR image of the lesion demonstrates marked enhancement following the IV administration of gadolinium due to the rich vascular nature of these lesions.. |
|
|
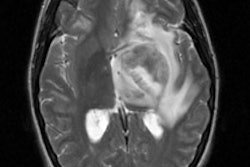
Astrocytomas were detected in about 70% of cases during clinical trials. Low grade astrocytomas (I and II) were best visualized, while more dedifferentiated lesions (grades III and IV) were demonstrated less frequently. This may in part be related to the large percentage of low grade tumors which express somatostatin receptors (80%), while such receptors are only rarely expressed on high grade lesions. Nonetheless, unless there is disruption of the blood brain barrier, Octreotide (a polar water soluble compound) will not accumulate in the tumor. Nonspecific Octreotide accumulation in somatostatin receptor negative CNS tumors can occur and is most likely related to disruption of the BBB [51, 52]
Virtually all meningiomas demonstrate somatostatin receptors - the major subtype overexpressed is SSTR 2 [50].. The detection of meningiomas (which are extra-axial lesions) was 100% in clinical trials and the intensity of the scintigraphic signal correlates well with the tumor sommatostatin receptor density [JNM, Mar 95, p.403-410].
Somatostatin receptors are expressed by both the normal
pituitary gland and pituitary tumors [50]. Research shows a
close correlation between the presence of somatostatin receptors
on growth hormone producing pituitary tumors and Octreotide
accumulation [50]. About 70% of growth hormone producing tumors
are identified. Non-functioning pituitary tumors also contain
somatostatin receptors and can be visualized in 75% of cases.
However, other authors suggest that nonsecreting adenomas
predominantly express SSTR 3 [50].Thyroid stimulating hormone
(TSH) secreting pituitary tumors can also be visualized with
nearly 100% sensitivity in clinical trials.
Hemagioblastoma:
CNS hemangioblastomas are cardinal features of von
Hippel-Lindau syndrome and occur in 60-80% of patients (the
cerebellum is the most common site) [50]. The tumor is known to
express SSTRs [50].
Medulloblastoma:
Medulloblastoma is the most common pediatric brain tumor with
the highest incidence between the ages of 3 to 9 years [50].
Medulloblastomas have been shown to express high levels of
SSTRs- most commonly SSTR 2 and 3 [50].
Supratentorial PNET:
Supratentorial PNETs account for 2.5% of brain tumors in
children [50]. The tumor can overexpress SSTRs - the
predominant receptor subtype is SSTR 2 (up to 100% of tumors)
[50].
The overall sensitivity for detecting Hodgkins lymphoma is between 70 up to 100%. Detection for supradiaphragmatic disease is superior to subdiaphragmatic lesions (88% vs. 13%). Sub-diaphragmatic lesion detection is hampered by the large amount of background activity. Differentiation from adenopathy secondary to granulomatous disease is not possible. The critical size for node detection is approximately 2 cm [53].
The overall detection of non-Hodgkins lymphoma is variable (sensitivity 35%-62%). Supradiaphragmatic abnormalities are better detected [16]. Detection of high grade lesions is superior to that of low grade tumors (44% vs. 29%). Detection of bone marrow involvement is poor. The role of Octreotide imaging in patients with NHL is limited [8,16].
Medullary thyroid carcinoma (MTC) arises from the parafollicular C-cells which secrete calcitonin. There is an association with the MEN syndromes, IIa and IIb. MTC's express and release carcinoembryonic antigen (CEA) in addition to calcitonin. Both CEA and calcitonin levels are used to monitor disease in these patients. Normalization of calcitonin levels following surgery is a strong indicator that the tumor was completely removed. Pronounced elevations in CEA are often associated with more aggressive tumors. Medullary carcinoma confined to the thyroid gland is potentially curable by total thyroidectomy. Unfortunately, metastases to local nodes are found in about 50% of patients at the time of diagnosis. In patients with metastatic disease, the prognosis is poor, with a 10 year survival of only about 30%.
The sensitivity of Octreotide for the detection of MTC is variable (41-71%) and may be related to loss of somatostatin receptors as the tumor becomes less differentiated. A higher ratio of calcitonin to CEA levels may also be associated with a greater likelihood for a positive exam [8]. The addition of Octreotide imaging to the detection of medullary thryoid carcinoma does not lead to a significant increase in metastatic lesion detection and octreotide probably has only a limited role in the management of these patients. [5, 10,11,12,31]
Merkel cells are distributed throughout normal skin and express a number of neuroendocrine characteristics. Merkel cell tumors are also referred to as neuroendocrine carcinomas of the skin and are aggressive tumors with a high incidence of spread to regional nodes (45-91%) and distant metasases (18-52%). Overall detection of tumor sites was 80% by Octreotide scintigraphy. Small lesions (0.5 cm) may go undetected [1].
The detection of neuroblastoma is about 90%. MIBG will detect some lesions not identified by Octreotide and visa versa. Patients with somatostatin receptor negative tumors generally have a worse prognosis [13].
Pancreatic Islet Cell Tumors (Pancreatic neuroendocrine tumors):
Pancreatic endocrine (PETs) or islet cell tumors arise from APUD cells and are hormonally active in 85% of cases. Tumors smaller than 0.5 cm are defined as microadenomas [42]. The lesions account for 1-2% of all pancreatic neoplasms [42]. Non-functioning tumors account for approximately half of all pancreatic endocrine tumors [42] (previously, non-functioning tumors were felt to account for approximately 15% of islet cell tumors and these tumors are typically very large with liver metastases at the time of presentation). Examples of syndromic endocrine tumors in decreasing order of frequency are insulinoma (60%), gastrinoma (20%), glucagonoma (3%), VIPoma (2%), and somatostatinoma (less than 1%) [37]. The presence of liver metastases is associated with a worse prognosis in patients with PETs [42].
Between 90 to 100% of islet cell tumors contain somatostatin receptors, while pancreatic adenocarcinomas never contain these receptors [36]. In general, these tumors behave in a malignant fashion, except for insulinomas, and metastatic disease is frequently present at the time of diagnosis. Islet cell tumors are associated with MEA I (Wermer's syndrome). There is also an association with von Hippel-Lindau syndrome [42]. Although VHL is frequently associated with serous cystadenomas of the pancreas (found in 17-56% of patients), islet cell tumors may occur in 5-17%- more commonly in VHL patients with pheochromocytomas [32,42]. VHL patients with sporadic PETs usually present at a younger age (mean 38 years) and are more likely to have multiple lesions (30% of cases)n[42]. In VHL patients, the frequency of malignancy and metastases is low (1-10%) and the tumors are typically non-functional [32,42].
In general, the lesions are hyperattenuating on arterial and venous phase images [42]. Larger lesions tend to be more heterogenenous due to areas of cystic degeneration and necrosis [42]. On MR, the tumors appear hypointense on T1 and have signal intensity higher than the pancreas on T2 images [42].
Insulinoma:
This is the most common functioning islet cell tumor- accounting for just over 40% of PETs [42]. It arises from the beta cells of the pancreas and produces hypoglycemia due to insulin secretion. Patients present with "Whipple's triad" which refers to symptoms of hypoglycemia (serum glucose< 40) such as dizziness or weakness associated with fasting or exercise that are relieved by glucose. There is a slight female predominance (1.4:1) and the mean age at presentation is 47 years [42]. Mostly insulinomas behave in a benign manner (90-94% [37]) and they are generally solitary (usually less than 2 cm in size). Insulinomas tend to manifest earlier and have a smaller size than other PETs due to the symptoms they produce [42]. The lesion is located with equal distribution in the head, body, and tail of the pancreas [37]. The tumor usually occurs sporadically, but they account for 10-30% of functioning PETs in patients with MEA I and in these situations, there may be multiple lesions. Insulinomas have also been reported in patients with neurofibromatosis type I [42]. Tretament is surgical excision.
On CT, the lesion is very vascular and will enhance with IV contrast. About 90% of insulinomas are smaller than 2 cm in size and 40% are smaller than 1 cm [37,42].
The reported sensitivity of Octreotide for the detection of insulinomas can be as low as 30 to 40%, which is lower than that for other islet cell tumors and probably the result of the absence of somatostatin receptors in these tumors [43], or a somatostatin receptor with a lower affinity for octreotide (when evaluating pancreatic endocrine tumors, Octreotide imaging has the lowest sensitivity for the detection of insulinoma and the highest for gastrinomas [42]). However, higher sensitivity for lesion detection (87.5%) has been reported with the use of 4 hour post injection SPECT images [6]. For SPECT imaging, an adequate dose of the radiotracer (about 250 MBq) and a long acquisition time per projection are essential for improved lesion detection [6].
Gastrinoma:
Gastrinoma is the second most commonly functioning islet cell tumor [42]. The peak incidence is in the 5th decade of life and there is a slight male predominance (1.3:1) [42]. Excess amounts of gastrin lead to stimulation and hyperplasia of the parietal cells in the gartic fundus which results in a 10 to 20 fold increase in gastric acid production. Patients present with intractable gastric ulcer disease. This is referred to as Zollinger-Ellison syndrome (Zollinger-Ellison syndrome accounts for only about 0.1% of cases of peptic ulcer disease [42]. Most of the ulcers are in the stomach and duodenum, but 25% will be in atypical sites such as the distal duodenum or proximal jejunum. Although one frequently associates this syndrome with the presence of multiple ulcers, there is a solitary ulcer in 90% of cases. Diarrhea is not uncommon and is caused by fat malabsorption secondary to the breakdown of pancreatic lipase from excessive gastric acid [31]. The diagnosis is established on the basis of elevated fasting serum gastrin levels (serum gastrin levels are often markedly elevated at over 1000 pg/mL (normal < 100 pg/mL) [31,42].
Previously, most gastrinomas were felt to arise from the pancreas, with approximately 10% located outside the pancreas- most commonly in the duodenum, but also in the stomach and splenic hilum. However, it is now felt that the lesion is more common in the duodenum - accounting for 80% of sporadic lesions and 90% of lesions associated with MENI [42]. Duodenal gastrinomas are usually small tumors measuring less than 1 cm in diameter [42]. The "gastrinoma triangle" (the sites accounting for the most common locations) is formed superiorly by the cystic duct confluence, medially by the the junction of the neck and body of the pancreas, and inferiorly by the junction of the second and 3rd parts of the duodenum [37,42]. When located in the pancreas, the most common location for a gastrinoma is the pancreatic head [42].
Most gastrinomas arise sporadically, however, they are the most common functioning PET in patients with MENI [42]. Underlying MEN I is seen in 20-25% of cases of gastrinoma [28,42]. Multiple tumors are found in about 10% of cases and this is associated with MEA I syndrome. Most gastrinomas are slow gorwing, but 50-70% of lesions behave in a malignant manner- i.e: have metastases at the time of diagnosis [31]. Treatment consists of total gastrectomy if the tumor cannot be located.
Octreotide has been shown to be highly sensitive in the detection of gastrinomas (60-93%) [8,31]. Better lesion detection is accomplished with the use of SPECT images and a higher dose of the agent (6 mCi) [8]. Results of Octreotide imaging can result in alterations in patient management in up to 47% of patients [8].
Glucagonoma:
This is a very rare tumor which arises from the alpha cells of the pancreatic islets- usually in the body or tail [31,42]. It is the third most common PET generally affecting patients between 40-60 years of age with an equal sex distribution [42]. The tumor is almost always sporadic and are only rarely associated with MENI [42]. The serum glucagon level is usually 10-20 times above normal [42]. Glucagon counteracts the effects of insulin on glucose metabolism resulting in glucose intolerance [31]. Patients present with hyperglycemia (diabetes), weight loss, anemia, and a migratory necrolytic erythema - a characteristic necrotizing skin rash consisting of painful pruitic plaques [42]. The rash is seen in two-thirds of patients and resolves when the serum glucagon level returns to normal) [31,42]. Nearly all patients have the skin rash at presentation- it typically begins at the groin and lower extremities (other authors indicate that it starts in the abdomen and groin and spreads to the trunk and extremities [42]), is highly pruritic, and migrates [31]. The clinical syndrome resulting from glucagonomas is referred to as the "four D's"- diabetes, dermatitis, deep vein thrombosis (and PE), and depression [31]. Other symptoms include diarrhea, glossitis, weight loss, and various neurologic and psychiatric symptoms [42]. Most glucagonomas behave in a malignant manner [42]. Metastases to the liver or lymph nodes are present at the time of diagnosis in over 50% of cases [31]. Octreotide has a sensitivity of 73% in tumor detection.
VIPoma: (Vasoactive intestinal peptide)
Vasoactive intestinal peptide (VIP) is a neuropeptide with a primary action of vasodilatation and inhibition of gastric acid secretion [31]. VIP acts on cyclic adenosine monophosphate within the bowel epithelium to inhibit the absorption of and stimulate the secretion of water and electrolytes into the bowel lumen [42]. Patients present with Verner-Morrison (WDHA) syndrome- severe watery diarrhea (6-8L per day), hypokalemia and achlorhydria [31,42]. The average duration of symptoms until diagnosis is about 3 years [14]. An elevated fasting serum VIP level >100 pg/mL is highly specific for VIPoma [42].
The lesion usually manifests in the 5th or 6th decades, but they have been reported in patients 2-83 years old [42]. VIPomas are rarely associated with MENI [42]. About 80% are malignant. The majority of VIPomas occur in the tail or body of the pancreas (60-90% of cases) [14,31]. Ectopic lesions can occur in about 10-20% of cases [37,42] in the duodenum, stomach, or small intestine. Metastases are frequently present (up to 50-80% of cases) at the time of diagnosis- typically to the liver, lymph nodes, and bone [14,31,42]. Octreotide sensitivity is about 86-88%, but sensitivity is much lower for lesions less than 1 cm in size [14,31].
Somatostatinoma:
Somatosatinomas are very rare lesions [42]. The mean age at presentation is 50 years and there is equal sex distribution. The lesion most frequently occurs in the pancreas (most commonly the pancreatic head) or periampullary region of the duodenum in up to 50% of cases (the lesion has also been reported in the small bowel, colon, and rectum) [37,42]. Dueodenal lesions are more likely to be associated with neurofibromatosis type I than are pancreatic lesions- 43% of patients with a duodenal stomatostatinoma have neurofibromatosis type I, compared to only 1.2% of patients with pancreatic lesions [42]. Somatostatin inhibits intestinal absorption and relaease of insulin, glucagon, gastrin, and pancreatic enzymes which may lead to diabetes, steatorrhea, diarrhea, cholelithiasis, hypochlorhydria, and weight loss [42]. Metastases are present in 50-75% of patients at the time of diagnosis [42].
|
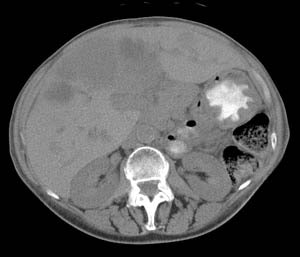
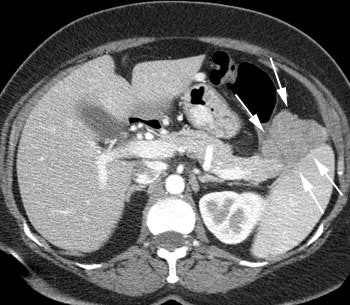
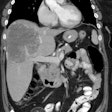
VIPoma: The patient shown below presented for evaluation of a 9 month history of intractable diarrhea and an elevated VIP level. Previous conventional imaging studies had been negative. The In-111 Octreotide study demonstrated a large area of intense uptake within the pancreatic tail (black arrow on transaxial SPECT images). Repeat CT imaging confirmed the presence of a VIPoma within the pancreatic tail (white arrows). |
|
|
Non-functional Pancreatic Neuroendocrine Tumor (Poorly differentiated neuroendocrine tumors):
Non-functioning islet cell tumors (also called non-functioning neuroendocrine tumors) that come to clinical attention are almost always malignant (80-90% are malignant) [35]. They are now felt to account for half of pancreatic endocrine tumors [42]. The lesion is usually sporadic, but they are the most common PET in patients with MENI and von Hippel-Lindau syndrome [42]. They are relatively slow growing and are usually quite large (over 6 cm) by the time of presentation. In many cases, malignancy can only be confirmed by identification of invasion of adjacent organs or the presence of distant metastases [35]. Metastases are found in 10% of cases and patients generally have relatively long survival [21], however, other authors suggest mets are present in 60-80% of patients at the time of diagnosis [42]. Patients with von Hippel-Lindau disease have an increased risk for non-functioning pancreatic neuroendocrine tumors (seen in about 5% of patients) [21]. Non-functioning tumors are frequently hypervascular. Central necrosis is common and calcification is seen in up to 25% of cases. Surgery is the only curative therapy for nonfunctioning pancreatic neuroendocrine tumors [35]. A feature of poorly differentiated neuroendocrine tumors is the absence or loss of somatostatin receptor activity so that these tumors cannot be reliably images with Pentetreotide [31]. However, they usually have a higher proliferative rate and can be imaged with FDG PET (the greater the degree of uptake, the worse the prognosis) [31].
The predominant somatostatin receptor subtypes expressed by pheochromocytomas are subtypes 3 and 4 [34]. Octreotide has only moderate affinity for these subtypes, compared to subtypes 2 and 5 [34]. Hence- results have been variable for imaging pheochromocytoma [34]. Overall, Octreotide sensitivity in detecting pheochromocytomas has been reported to be between 86 and 100% (with higher sensitivity for detecting metastatic pheo than benign pheo- sensitivity 91% versus 28.5%, respectively [34]). Small lesions may be difficult to detect secondary to tracer uptake in the liver, spleen, and kidneys.
In a direct comparison of Octreotide to MIBG imaging in patients with malignant pheochromocytoma both were found to have a similar sensitivity (86-93% for Octreotide, and 86-88% for MIBG). However, the contrast and intensity of uptake were higher with MIBG, MIBG detected more lesions, and MIBG was more sensitive for the detection of bone and hepatic metastases. Octreotide detected the presence of lung mets in a patient that had a negative MIBG exam, but the intensity was really cranked up on the Octreotide image and a similar intensity adjustment of the MIBG exam was not made. Another drawback of the study was that comparison was made to both diagnostic I-123 MIBG scans and post-therapeutic I-131 MIBG studies [1,2,9]. In another study, Octreotide detected 87% of metastatic lesions, while I123 MIBG detected 57% [34]. Other authors report that Octreotide is less sensitive than MIBG for the evaluation of pheochromocytoma (sensitivity as low as 28.5% for non-metastatic pheo [34]) with false-negative rates as high as 66-75% [31,45].
Based upon the available literature- Somatostatin imaging is best reserved for patients with suspected metastatic pheo. In general, patients with predominantly Octreotide positive lesions have rapidly progressive tumors [34].
Granulomatous Disorders:
Uptake of Octreotide has also been reported in granulomatous disorders such as sarcoid, Wegener's, and tuberculosis [15].
Idiopathic pulmonary fibrosis:
Octreotide uptake has also been reported in pulmonary fibrosis and correlates with the intensity of the alveolitis [30].
Radionuclide Therapy of neuroendocrine
tumors (PRRT):
In cases of metastatic disease, treatment options may include streptozocin-based chemotherapy, chemotherapy with capecitabine and temozolomide, and peptide receptor radionuclide therapy (PPRT) [59]. Patients with predominant liver disease can also receive liver directed therapies such as debulking surgery, embolization, chemoembolization, radioembolization, or RFA [59]. Newer treatment options include sunitinib (Sutent- a tyrosine kinase inhibitor) or everolimus (Afinitor- an inhibitor of mammalian target of racamycin) [59].
High dose 111In-pentetreotide:
High dose 111In-pentetreotide can be used to treat patients with disseminated neuroendocrine tumors (peptide receptor radiotherapy or PRRT) [22]. 111In produces an Auger electron with an energy less than 30keV and range of about 80-200 nm, and an internal conversion electron with a range of 200-500 um [22,23]. These emissions can have an anti-tumor effect when the agent is internalized into the cells cytoplasm and particularly when they occur near the cell nucleus [22,23]. The administered activity is typically between 3 to 5 GBq (containing about 30 ug of pentetreotide) [22]. Up to 70% of patients will demonstrate some benefit (objective response or or tumor stability) from the treatment without any significant toxicity [22]. The response may be related to tumor burden, so that any given amount of radiation will be spread among more tumor if there is a large tumor bulk thereby reducing the amount of radiation per gram of tumor [22]. The compound is cleared via the kidneys and resorbed and partially retained in the proximal tubules- causing dose limiting nephrotoxicity [40]. Myelodysplastic syndrome or leukemia has been reported in 50% of patients who received very high dose therapy (>100 GBq) [61].
90Y and 177Lu are both beta emitters that can be used for PRRT therapy [60]. 177Lu has a half-life of 6.7 days and particle range of 2 mm [60]. The agent also emits gamma rays that allow post-treatment imaging and dosimetry calculations [60]. 90Y has a half-life of 64.1 hours and a particle range on the order of 12 mm [60].
177Lu-octreotate and 90Y-octreotide (PRRT) [56]. 177Lu-octreotate exhibits greater somatostatin receptor 2 affinity [56]. A survival impact and objective responses (partial and complete) have been described in up to 30% of patients receiving PRRT [56]. Acute hematologic toxicity is mild and transient in approximately 90% of patients [56]. Myeloproliferative events are rare (1-2%) [56]. The kidneys represent the critical organ- especially with 90Y-octreotide [56].
Other radiolabeled SSA's:
90Y-DOTATOC and 177Lu-DOTATATE allow delivery of high-absorbed doses to tumors expressing SSR2 recptors [38]. Y90 has a half-life of 2.7 days, an energy of 935 keV, a path length of 12 mm in soft tissue, and no gamma emission [63]. Lu177 has a half-life of 6.7 days, a mean effective blood elimination half-life of 3.5 +/- 1.4 hours, a mean terminal blood half-life of 71 +/- 28 hours, an energy of 133 keV, a path length of 2mm in soft tissue, and gamma emissions (which permit imaging) of 113 keV (6.6%) and 208 keV (11%) [63,65]. Because Y90 releases more energy and has more tissue penetration it is presumed to be better choice for bulky tumors [62]. Unfortunately, the longer path length can also result in greater toxicity to surrounding normal tissue, such as the bone marrow and kidneys [63]. 90Y-DOTATOC treatment was associated with partial response rates of 25-30% in adults [41] and 177Lu-DOTATATE treated patients showing complete or partial remission in 30% and minor response in 16% [57]. For both 90Y-DOTATOC and 177Lu-DOTATATE, the critical organ is the kidney followed by the bone marrow [41,57]. Because of it's potential for lower toxicity, 177Lu-DOTATATE was selected for commercialization [63].
Eligible patients include those with inoperable or metastatic NET with disease progression on standard dose SSTR-therapy with either octreotide or lanreotide [63,66]. Patients with midgut NETs, pancreatic NETs, and NETs of unknown primary are considered the most appropriate for PRRT therapy [120]. Patients considered for treatment should have prior SSTR-based imaging demonstrating sufficient tracer tumor uptake (greater than the liver) [66]. Patients should also have a serum creatinine level less than 1.7 mg/dL (or a creatinine clearance > 50 mL/min), hemoglobin level greater than 8 g/dL, WBC count greater than 2000, platelet count greater than 75,000, bilirubin level less than 3 times the upper limit of normal, and a serum albumin level greater than 3 g/dL, unless the prothrombin time is within the normal range [63]. Other authors indicate that they do not consider a GFR < 50 to be a contraindication to treatment (as long as an amino acid infusion is per performed), but patients with a GFR < 30 should receive therapy only in exceptional circumstances [68]. Additionally, any hydronephrosis should be corrected prior to treatment [120]. Eligible patients should have a Karnofsky performance status greater than 50, and expected survival longer than 3 months [66]. In 177Lu-DOTATATE therapy, patients receive repeated cycles of 7,400 MBq (200 mCi) of the agent [58] (treatment is given every 8 weeks and patients receive a total of 4 doses [66]).
Long acting SSAs should not be administered for at least 4 weeks before the therapy, but may be administered between 4 and 24 hours after each 177Lu-DOTATATE dose [66]. Short acting SSAs can be given up to 24 hours before therapy if the patient has symptoms [66].
The agent is administered IV over 30 minutes followed by a 10-20 minute infusion of saline solution [63]. The agent must be given in conjunction with an amino acid formulation which is administered IV through either the same or a separate IV line [63]. Other authors indicate that the amino acid infusion should be initiated 30 minutes before the 177Lu-DOTATATE infusion, continued through the treatment infusion and for at least 3 hours afterward [66]. A peripheral vein in the antecubital fossa is the preferred location for venous access, and intravenous lines in both arms are preferred [65]. Central lines may be used for the administration of the premedications and the amino acid solution if there is difficult peripheral venous access, however, a peripheral line is the recommend route for the administration of the 177Lu-DOTATATE infusion, as the use of central lines has not be studied [65]. Should extravasation occur, the infusion should be stopped immediately [66]. Aspiration of the extravasated agent can be considered [66]. Warming the region to accelerate vasodilation, applying massage, and elevating the arm are helpful short-term management strategies [66].
Amino acids reduce the residence time of 177Lu-DOTATATE in the kidneys by decreasing the resorption of the agent via the proximal tubules, thereby reducing renal radiation toxicity [63,65]. The only two key amino acids for the solution are positively charged lysine and arginine [63,66]. High concentration commercial amino acid solutions typically include additional amino acids which raise the osmolality of the solution and are associated with significant nausea and vomiting during the infusion [65]. Commercial amino acid solutions should be used only if they have an osmolarity less than 1050 mOsmol [66]. Purer formulations with a lower osmolality solution help to reduce nausea associated with the infusion [63]. Electrolyte imbalances, mostly hypokalemia, can also occur, especialyl following infusion of concentrated amino acid solutions [66].
The target infusion rate of the commercial amino acid solutions should reach 320 mL/h and 177Lu-DOTATATE should generally not be administered until this rate is reached or until one eighth of the total volume of amino acid solution has been infused [65]. The amino acid solution should infuse concurrently with the 177Lu-DOTATATE, and continue at 320 mL/h or greater until the total volume has been administered [65]. To decrease nausea and vomiting, the high concentration amino acid solution infusion can begin at a low rate of 100 mL/h and be increased slowly by 20-50 mL every 15-20 min [65]. An alternative compounded amino acid formulation consisting of solely 25 g of lysine and 25 g of arginine diluted in 1L of normal saline for injection is substantially less emetogenic and can be infused over a shorter period of time (250 mL/h for 4 hours commencing 30 minutes before treatment with the radiopharmaceutical) [65].
Nausea and vomiting are common during the administration of commercial amino acid solutions when infusion rates are above 250 mL/h [65]. Therefore, it is recommended to use an IV premedication regimen consisting of a 5-HT3 antagonist (such as granisetron, ondansetron, or palonosetron), an NK1 receptor antagonist (such as fosaprepitant), and an H2 receptor antagonist (famotidine) [65]. Patients that receive compounded arginine-lysine generally require only a 5-HT3 antagonist as prophylaxis [65].
Therapy is contraindicated in pregnancy and breast feeding should be stopped for treatment and not restarted until 2.5 months following the final therapy [65]. Pregnancy should be avoided for 6 months after completion of the final treatment [65].
The number of cycles is determined on the basis of the biologically effective dose delivered to the kidney (the principle organ at risk) [58]. The maximum accepted doses are 23-25 Gy to the kidneys and 2 Gy to the bone marrow [41,46,57].
Radiation risks:
The emitted radiation immediately after administration of 177Lu-DOTATATE is 2 mR/hr at 1 meter (1.8 +/- 0.5 mrem/h) [63,65,66]. Within 24 hours, this will decrease to less than 1 mR/hr at 1 meter [63,66]. Therefore, patients can be treated on an outpatient basis [63]. The patient should be encouraged to maintain good hydration, exercise good bathroom hygiene, and avoid children, pregnant women, and crowds for approximately 3 days [63]. 177Lu-DOTATATE is primarily excreted in the urine, with a cumulative excretion of 44% within 5 hours, 58% within 24 hours, and 65% within 48 hours after administration [65]. Some authors suggest that the in vivo stability of 177Lu-DOTATATE is lower than previously assumed with a major part of radioactivity in the plasma consisting of 177Lu-labeled metabolites by 24 hours [69]. These metabolites appear to be removed from the body by hepatic clearance and not by renal excretion [69].
Results of PRRT therapy:
177Lu-DOTA has been show to be an option for neoadjuvant treatment in patients with unresectable pancreatic neuroendocrine tumors, potentially leading to successful resection surgery [59]. 177Lu-DOTATATE can result in markedly longer progression free survival with significant myelosuppression observed in fewer than 10% of patients [60]. In one study, the rate of progression free survival at 20 months was 65% for the patients treated with 177Lu-DOTATATE versus 11% in the control group [60]. Compared to standard long-acting octreotide treatment, PRRT has been shown to improve progression free survival- a 79% lower risk of disease progression [66]. Re-treatment can be performed as salvage therapy, but PFS survival is shorter compared to previously untreated patients (approximately 14-15 months).
The therapy is most commonly given as 4 cycles with 2 months between treatments [69]. Initial response assessment is typically done 1 month after the second cycle of therapy [63]. At the first assessment if there is any outcome but disease progression, therapy is continued [63]. Response is again assessed 1 month following completion of all 4 cycles of therapy [63]. Following successful treatment, CT imaging may demonstrate increase in size or development of new bone lesions [47]. Correlation with SRS imaging is important to show that the lesions demonstrate decreased tracer uptake indicative of treatment response [47]. Patients that demonstrate disease early progression following treatment have a poor prognosis [64].
A more favorable response to PRRT therapy is seen in patients with a low number of disease sites [64].
Factors associated with lower progression free survival following PRRT include a Ki-67 of more than 5%, previous treatment with interferon-alpha and chemotherapy, diabetes, and chromograninA (CgA) levels higher than 336 ug/L [67]. Factors associated with lower overall survival include a Ki-67 of more than 10%, previous chemotherapy and ablation, and CgA levels higher than 112 ug/L [67].
Complications:
Renal toxicity: Renal failure can be observed in patients that receive up to 7.4 GBq/m2 in the absence of kidney protection with cationic amino acid infusions [41]. By using a cationic amino acid infusion (lysine and arginine) for 30 minutes before and continuing for 90 minutes after the treatment (combined with limiting the renal dose to less than 25 Gy) can reduce renal uptake of radiolabeled somatostatin analogs by 40% and has been shown to considerably reduce renal toxicity [41,46]. Radioprotective drugs such as amifostine are also being explored to aid in decreasing renal toxicity [46]. Individualized absorbed dose calculations can be performed to determine the maximum safe dose a patient can receive- thereby maximizing the therapeutic effects of the treatment, while limiting critical organ toxicity [48]. DOTATOC has exhibited the lowest normal organ dose with the best tumor-to-kidney ratio [60]. A long term follow-up of PRRT treated patients demonstrated non-dialysis dependent chronic renal toxicity in combination with anemia in up to almost two-thirds of treated patients [64]. Other authors suggest long-term grade 3-4 renal toxicity is rare and seen in less than 2% of patients, but patients with long-standing diabetes or longstanding HTN may be at greater risk after therapy [65,66]. Another risk factor is renal outflow obstruction [66].
Hemotoxicity: Bone marrow suppression is the most common delayed side effect of PRRT, typically seen 4-8 weeks after treatment (thrombocytopenia is the most common hematotoxicity) [66]. It is also one of the more serious side effects following 177Lu-PRRT therapy. Subacute reversible grade 3-4 hemotologic toxicity can be seen in 11.3% of patients (the mean time to blood count recovery is 12 months after termination of PRRT) [49,61]. Other authors indicate grade 3 and 4 thrombocytopenia and neutropenia occur in
Treatment related cancer: Persistent hematologic dysfunction can be seen in up to 4% of patients- including bone marrow failure (1% of patients) and hematologic malignancy (1-4% of patients; acute leukemia (<1%) and myelodysplastic syndrome (< 1.4%)) with a median of 2 years after therapy (other authors state a mean latency for hematologic malignancy of more than 40 months [66]) [49,61,63,65,66]. The median time for persistent hematolgic dysfunction was 41 months following the first cycle of PRRT [61]. Age older than 70 years, baseline cytopenia, presence of bone metastases, large number of prior therapies, prior treatment with alkylating agents, and prior radiotherapy have been reported to increase the risk for secondary myelodysplastic syndrome [66]. In re-treated patients, the cumulative incidence of myelodysplastic syndrome and acute myeloid leukemia in retreated patients was reported to be 2.2% [66].
To avoid bone marrow hypoplasia, a maximum absorbed dose to the bone marrow of 2 Gy has been suggested [49]. Some authors suggest risk factors for MDS and leukemia include previous chemotherapy, tumor invasion of the BM, and prior myelotoxic therapies [61].
Neuroendocrine hormonal crisis (carcinoid crisis): Due to excessive release of hormones or bioactive substances crisis develops in 1% of patients and typically occurs during treatment or within two days of initial treatment [65]. Patients with high tumor loads, liver metastases, high levels of chromogranin A and urinary 5-hydroxyindoleacetic acid, and established carcinoid syndrome or known carcinoid heart disease are at a higher risk of neuroendocrine hormonal crisis during PRRT [66]. A subcutaneous bolus of dose of 250-500 ug of octreotide can be given within 1-2 hours before the treatment as prophylaxis, but crisis can still occur [66]. Typical clinical manifestations include cutaneous flushing, diarrhea, bronchospasm, and hypertension [65]. Hormonal crisis can be treated with IV high-dose SSAs (for hypotension or life threatening symptoms during or immediately after PRRT IV bolus doses of 500-1000 ug octreotide are advised, with treatment repetition at 5 minute intervals until control of symptoms. Alternatively, after an initial IV bolus, continuous octreotide infusion may be performed at 50-100 ug/hr), IV fluids, corticosteroids (IV dexamethasone 4-8 mg), and correction of electrolyte disturbances [65,66]. Other authors indicate that hormone crisis can occur in 1-10% of patients either during or within a few days of PRRT [63].
Other side effects: Patients with underlying mesenteric and peritoneal disease may be at increased risk for developing bowel obstruction following treatment- possibly related to an inflammatory/fibrotic response to therapy (frozen abdomen due to adhesions) [70]. Mild hair loss, asthenia, and decreased appetite are other reported long term side effects [66].
177Lu-Octreotate has been studied for treatment of bone metastases in patients with gastroenteropancreatic neuroendocrine tumors with promising results [44].
High dose I-131 MIBG:
PRRT therapy with 117Lu- or 90Y-based compounds yield higher morphologic response rates in carcinoid tumors (compared to I-131 MIBG therapy), renal toxicity is a potential significant complication [54]. High dose I-131 has also been used in the treatment of patients with metastatic carcinoid tumors [54]. One dose regimen consists of treatment doses of 11.1 GBq (300 mCi) per course with a minimium of 3 months between treatments [54]. The therapy has been shown to provide effective symptom palliation and disease stabilization with limited toxicity [54]. The primary side effects are transient myelosuppression (grade 3 or 4 in 15% of patients) and thrombocytopenia (7.6% of patients) [54]. Myelodysplastic syndrome has been observed in up to 3% of patients following cummulative doses of 66.6 GBq (1800 mCi) [54].
99mTc-HYNIC-Octreotide is a technetium agent that has
been developed for imaging neuroendocrine tumors [55].
REFERENCES:
(1) Eur J Nucl Med 1993; Krenning EP, et al. Somatostatin receptor
scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide:
the Rotterdam experience with more than 1000 patients. 20(8):
716-31
(2) Semin Nucl Med. 1995; Olsen JO, et al. Somatostatin receptor imaging of neuroendocrine tumors with indium-111 pentetreotide (Octreoscan). 25(3): 251-61
(3) J Nucl Med 1995; Kahaly G, et al. Indium-111-pentetreotide scintigraphy in Graves' ophthalmopathy. 36: 550-54
(4) J Nucl Med 1998; Reisinger I, et al. Somatostatin receptor scintigraphy in small-cell lung cancer: Results of a multicenter study. 39: 224-227
(5) J Nucl Med 1996; Kwekkeboom DJ, et al. Cost-effectiveness analysis of somatostatin receptor scintigraphy. 37: 886-892
(6) J Nucl Med 2000; Schillaci O, et al. 111 In-pentetreotide scintigraphy in the detection of insulinomas: Importance of SPECT imaging. 41: 459-462
(7) J Nucl Med 2000; Janson ET, et al. Nuclear localization of 111In after intravenous inhection of [111In-DTPA-D-Phe1]-Octreotide in patients with neuroendocrine tumors. 41: 1514-1518
(8) J Nucl Med 2000; Kwekkeboom D, et al. Peptide receptor imaging and therapy. 41: 1704-1713
(9) J Nucl Med 1995; Tenenbaum F, et al. Comparison of radiolabeled octreotide and meta-iodobenzylguanidine (MIBG) scintigraphy in malignant pheochromocytoma. 36: 1-6
(10) Nucl Med 1996; Juweid M, et al Radioimmunotherapy of medullary thyroid cancer with iodine-131 labeled anti-CEA antibodies. 37: 905-911
(11) Nucl Med 1996; Baudin E, et al. Comparison of octreotide scintigraphy and conventional imaging in medullary thyroid carcinoma. 37: 912-916
(12) J Nucl Med 1998; Kurtaran A, et al. Indium-111-DTPA-D-Phe-1-Octreotide and Technetium-99m-(V)-dimercaptosuccinic acid scanning in the preoperative staging of medullary thryoid carcinoma. 39: 1907-1909
(13) J Nucl Med 1996; Manil L, e tal. Indium-111-pentetreotide scintigraphy in children with neuro-blast derived tumors. 37: 893-896
(14) Clin Nucl Med 2000; Thomason JWW, et al. Somatostatin receptor scinitgraphy. The definitive technique for characterizing vasoactive intestinal peptide-secreting tumors. 25: 661-664
(15) J Nucl Med 2001; Lebtahi R, et al. Somatostatin receptor scintigraphy and gallium scintigraphy in patients with sarcoidosis. 42: 21-26
(16) J Nucl Med 2001; Lugtenburg PJ, et al. Somatostatin receptor scintigraphy in the initial staging of low-grade non-Hodgkin's lymphomas. 42: 222-229
(17) Applied Radiology 2001; Hanson MW. Scintigraphic evaluation of neuroendocrine tumors. 30: 11-17
(18) Radiology 2001; Hoegerle S, et al. Whole-body 18F DOPA PET for detection of gastrointestinal carcinoid tumors. 220: 373-380
(19) J Nucl Med 1993; Kweekkeboom DJ, et al. Octreotide scintigraphy for the detection of paragangliomas. 34: 873-878
(20) Laryngoscope 1998; Myssiorek D, Palestro CJ. 111Indium pentetreotide scan detection of familial paragangliomas. 108: 228-31
(21) Radiology 2002; Marcos HB, et al. Neuroendocrine tumors of the pancreas in von Hippel-Lindau disease: spectrum of appearances at CT and MR imaging with histopathologic comparison. 225: 751-758
(22) J Nucl med 2003; Buscombe JR, et al. Long term efficacy of high-activity 111In-pentetreotide therapy in patients with disseminated neuroendocrine tumors. 44: 1-6
(23) J Nucl Med 2003; Capello A, et al. Peptide receptor radionuclide therapy in vitro using [111In-DTPA0] octreotide. 44: 98-104
(24) J Nucl Med 2003; Schillaci O, et al. Somatostatin receptor scintigraphy in liver metastasis detection from gastroenteropancreatic neuroendocrine tumors. 44: 359-368
(25) J Nucl Med 2003; Duet M, et al. Clinical impact of somatostatin receptor scintigraphy in the management of paragangliomas of the head and neck. 44: 1767-1774
(26) J Nucl Med 2006; Ezziddin S, et al. Factors predicting tracer uptake in somatostatin receptor and MIBG scintigraphy of metastatic gastroenteropancreatic neuroendocrine tumors. 47: 223-233
(27) J Nucl Med 2006; Vegt E, e tal. Renal uptake of radiolabeled Octreotide in human subjects is efficiently inhibited by succinylated gelatin. 47: 432-436
(28) Radiographics 2006; Scarsbrook AF, et al. Multiple endocrine neoplasia: spectrum of radiologic appearances and discussion of a multitechnique imaging approach. 26: 433-451
(29) AJR 2006; Lee KY, et al. Extraadrenal paragangliomas of the body: imaging features. 187: 492-504
(30) J Nucl Med 2006; Lebtahi R, et al. Increased uptake of 111In-octreotide in idiopathic pulmonary fibrosis. 47: 1281-1287
(31) Radiographics 2007; Intenzo CM, et al. Scintigraphic imaging of body neuroendocrine tumors. 27: 1355-1369
(32) Radiographics 2008; Leung RS, et al. Imaging features of von Hippel-Lindau disease. 28: 65-79
(33) J Nucl Med 2008; Koopmans KP, et al. 111In-octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. 49: 1232-1237
(34) J Nucl Med 2008; Ilias I, et al. Comparison of 6-18F-Fluorodopamine PET with 123I-metaiodobenzylguanidine and 111In-pentreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. 49: 1613-1619
(35) AJR 2009; Malago R, et al. Contrast-enhanced sonography of nonfunctioning pancreatic neuroendocrine tumors. 192: 424-430
(36) J Nucl Med 1995; Apr., p.547
(37) J Nucl Med 2009; Sahni VA, Mortele KJ. The bloody pancreas: MDCT and MRI features of hypervascular and hemorrhagic pancreatic conditions. 192: 923-935
(38) J Nucl Med 2010; Ambrosini V, et al. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. 51: 669-673
(39) J Nucl Med 2010; Binderup T, et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scinitgraphy, and 18F-FDG PET. 51: 704-712
(40) J Nucl Med 2010; Vegt E, et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. 51: 1049-1058
(41) J Nucl Med 2010; Menda Y, et al. Phase I trial of 90Y-DOTATOC therapy in children and young adults with refractory solid tumors that express somatostatin receptors. 51: 1524-1531
(42) Radiographics 2010; Lewis RB, et al. Pancreatic endocrine
tumors: radiologic-clinicopathologic correlation. 30: 1445-1464
(43) Radiographics 2011; Low G, et al. Multimodality imaging of
neoplastic and nonneoplastic solid lesions of the pancreas. 31:
993-1015
(44) J Nucl Med 2011; Ezziddin S, et al. Response and long-term
control of bone metatases after peptide receptor radionuclide
therapy with 177Lu-Octreotate. 52: 1197-1203
(45) J Nucl Med 2012; Taieb D, et al. Modern nuclear imaging for
paragangliomas: beyond SPECT. 53: 264-274
(46) J Nucl Med 2012; Melis M, et al. Reduction of renal uptake
of radiolabeled octreotide by amifostine coadministration. 53:
749-753
(47) J Nucl Med 2012; van Vliet EI, et al. Tumor Response
Assessment to Treatment with [177Lu-DOTA0,Tyr3]Octreotate in
Patients with Gastroenteropancreatic and Bronchial Neuroendocrine
Tumors: Differential Response of Bone Versus Soft-Tissue Lesions.
53: 1359-1366
(48) J Nucl Med 2013; Sandstrom M, et al. Individualized
dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate
treatment.
54: 33-41
(49) J Nucl Med 2013; Sabet A, et al. Long-term hematotoxicity
after peptide receptor radionuclide therapy with 177Lu-Octreotate.
54: 1857-1861
(50) AJR 2013; Sharma P, et al. Somatostatin receptor-based
PET/CT of intracranial tumors: a potential area of application for
68Ga-DOTA peptides. 201: 1340-1347
(51) J Nucl Med 1995; Haldemann AP, et al. Somatostatin receptor
scintigraphy in central nervous system tumors. 36: 403-410
(52) J Nucl Med 1995; Lee JD, et al, Indium-111-Pentetreotide Imaging in Intra-axial Brain Tumors: Comparison with Thallium-201 SPECT and MRI
Read More: http://www.ajronline.org/doi/abs/10.2214/AJR.13.10897