Thyroid Cancer:
General:
I-131 scanning has been the mainstay for the evaluation of recurrent thyroid carcinoma. Patients who are found to have I-131 accumulating local recurrences or metastases on diagnostic scans will usually receive subsequent therapeutic I-131 doses which may be curative. Unfortunately, not all recurrences are iodine avid due to progressive tumor dedifferentiation [14] (50-60% of papillary and 64-67% of follicular cancer recurrences are iodine avid [4]). Patients with elevated human thyroglobulin levels (post TSH stimulation value greater than 10 ug/L), but negative I-131 scans pose a diagnostic and therapeutic dilemma [21]. Although therapy with I-131 has been performed in these patients with subsequent decline in thyroglobulin levels [5,22], one can never be certain if all sites of disease have been adequately treated. Surgical resection of untreated sites of disease would certainly be desirable and is the only curative therapeutic option available [4,16]. Other agents such as Tc-sestamibi, Tc-tetrofosmin, and thallium-201 have all be used to evaluate for non-iodine avid recurrent cancer and metastatic disease. Although useful for locoregional disease, physiologic accumulation of these agents in other organs (particularly within the abdomen) limits their usefulness for whole body examinations.
FDG PET imaging can aid in the detection of metastatic disease in post-thyroidectomy/I-131 ablation patients with negative diagnostic I-131 imaging [1,2,21]. Unlike conventional imaging, FDG PET imaging can detect metastatic disease to normal sized lymph nodes [1]. I-131 scintigraphy and FDG PET imaging are actually complementary diagnostic methods for the evaluation of recurrent or metastatic thyroid cancer [3,6]. The iodine uptake of a tumor is inversely related to its glucose metabolism [3]. Tumor uptake is also related to the degree of tumor differentiation [4]. In general, well differentiated thyroid carcinoma will be I-131 positive and FDG PET negative, while less differentiated cancers will show the reverse (i.e.: FDG PET positive and I-131 negative) [1,6]. This is likely related to a decrease in sodium-iodide symporter expression and an increase in glucose transporter-1 expression [26].
It is important to remember that although FDG-positive recurrences may indicate dedifferentiated and more aggressive tumors, this finding does not automatically predict a worse prognosis [4]. Another benefit of FDG PET imaging is the identification of unsuspected secondary malignancies [4].
One of the benefits of FDG PET imaging is that patients do not
necessarily need to discontinue hormonal replacement therapy prior
to imaging, however, lesion detection is improved when performing
the exam during TSH stimulation (following exogenous thyroid
hormone removal) [3] or following rhTSH stimulation [12,33]. It is
believed that increased TSH levels stimulate FDG uptake by thyroid
cancer cells which results in the identification of more lesions
[14]. A meta-anaylsis has shown that PET scans under TSH
stimulation result in significantly improved detection of thyroid
cancer mets [32].
In one study, FDG imaging was shown to be superior to FLT for the
evaluation of metastatic disease and tumor recurrence [30].
I-124 for the evaluation of
thyroid cancer:
I-124 (half-life 4.18 days) is a positron emitting agent which
may hold promise for imaging differentiated thyroid cancer. I-124
would allow for the accurate determination of the concentration of
the agent within a specific volume that could be used to determine
individual lesion dosimetry [13,19]. The positron abundance for
I-124 is only 23% with a mean positron energy 819 keV (max energy
2138 keV) [19]. The agent also emits photons with energies of 602
keV (60% abundance), 722 keV (10% abundance), and 1691 keV (11%
abundance)- these photons can affect PET imaging [19]. The 602 keV
photon can produce gamma-coincidences with the annihilation photon
and result in increased background noise [19]. The higher gamma
emissions can result in an increased singles rate (random
coincidences) [19]. Also- the presence of I-124 near the trachea
can cause a phenomenon that could be considered a "shin-through"
artifact [27]. The effect is due to high-energy positrons that
cross the air-filled trachea and annihilate at the opposite
tracheal wall- incorrectly suggesting uptake in that location
[27]. This phenomenon can be seen in up to 17% of patients that
undergo I-124 PET/CT imaging after thyroidectomy [27].
For imaging, a dose of approximately 1.7 - 2 mCi is used [28,34].
Imaging is performed 48 hours following tracer administration
[28]. Compared to I-131 imaging, in patients with suspected
metastases, I-124 can detect more foci of metastatic disease [28].
However, other authors suggest that compared to post-therapeutic
I-131 scans, pre-therapy I-124 imaging can result in false
negative results [34].
In patients that have undergone prior ablation in whom metastatic
disease is suspect, I-124 imaging should be performed following
thyroid hormone withdrawal, as opposed to thyrogen stimulation, as
a significantly greater number of metastatic foci will be detected
(fewer false negative exams) [28,35]. This is likely related to
increased tracer uptake by thyroid tissue which can be up to 23
times higher following hormone withdrawal compared to rhTSH
stimulation [35].
Applications for FDG PET in thyroid cancer:
Diagnosis:
FDG PET is probably not indicated for the diagnosis of thyroid cancer. Not all malignant thyroid lesions will accumulate FDG and some benign lesions will show increased metabolic activity. Therefore, FDG PET should not be considered a substitute for I-123 or I-131 whole-body scintigraphy [14]. However, focal thyroid uptake identified on PET imaging performed for other reasons should be further evaluated as there is a high likelihood (30-50%) for an underlying primary thyroid cancer [21].
Staging:
No information is currently available regarding the utility of FDG PET for the staging of thyroid carcinoma. It will likely not play a primary role in the initial staging of thyroid cancer.
Metastatic disease:
In a comparison of FDG PET to bone scan for the detection of bone metastases the sensitivity of PET was 85% compared to 78% for bone scan [25]. Although this difference was not statistically significant, there were fewer false positive PET exams and PET was overall more accurate (98%) [25].
FDG PET Imaging in Patients with Suspected Recurrent Thyroid Cancer and Negative I-131 Scans:
Negative whole body iodine imaging can occur in 10-15% of patients with elevated thyroglobulin levels- either the result of small volume disease or tumor de-differentiation [20]. The American Thyroid Association recommends that FDG PET imaging be considered to evaluate high-risk patients with elevated serum Tg levels (generally unstimulated > 10 ng/mL) with negative findings on RAI imaging [33,37]. However, other authors disagree with this cutoff and point out that as thyroid cancer cells dedifferentiate they have a reduced capacity to produce and secrete thyroglobulin (and that a low thyroglobulin level therefore does not indicate a small tumor burden) [33].
For the detection of recurrent thyroid cancer in patients with negative I-131 scans, FDG PET imaging has a reported sensitivity of 68-94%, specificity of 42-95%, and accuracy of 74% [1,4,7,14,20,23,30]. Interestingly, patients with higher thyroglobulin levels (above 100 ug/L) have been shown to have a higher incidence of true-positive FDG PET exams [4]. In one study that evaluated the sensitivity of PET in relation to the thyroglobulin level- sensitivity was 60% for Tg less than 5, 63% for Tg between 5-10, and 72-83% for Tg level more than 10 ng/mL - suggesting that higher diagnostic accuracy with higher thyroglobulin levels [20,26,33]. FDG PET can detect the presence of metastatic disease in up to 70% normal sized lymph nodes [7]. Metastases to nodes as small as 4 mm have been demonstrated on PET imaging [7]. False positive exams have been reported in association with reactive lymph nodes, parathyroid adenomas, and infection [20].
FDG PET imaging can have significant impact on patient management in this difficult subset of patients. Treatment can be changed in 32-54% of patients based upon FDG PET imaging results, including change in surgical management (14-38%) [4,8,26]. A drawback of FDG PET imaging is that diffuse small lung metastases may not be identified and false negative exams can occur even in the presence of nodal metastases [7].
Since detection of sites of disease can be obscured by muscular uptake in the neck, it is important that muscle relaxants be used prior to scanning patients for recurrent thyroid cancer if possible [11].
PET/CT has been found to have an improved overall diagnostic accuracy (up to 93%) when compared to PET imaging alone [16]. Part of this improvement is related to improved localization of tracer uptake that permits proper identification of non-pathologic sites of tracer accumulation [16]. Another benefit of PET/CT is the ability of the CT portion of the examination to detect small lung metastases that would otherwise be missed on PET imaging [16]. Combined PET/CT imaging can result in a change in patient management in 44-48% of patients [16,20].
PET imaging can also be performed concurrently with I-131 therapy in patients with recurrent thyroid cancer or those with high risk tumors and has been shown to detect additional recurrent or metastatic lesions not seen on the post-therapy I-131 scan [31]. FDG PET imaging should be viewed as another tool for assisting in the detection of non-iodine avid thyroid metastases.
|
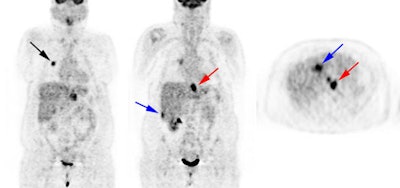
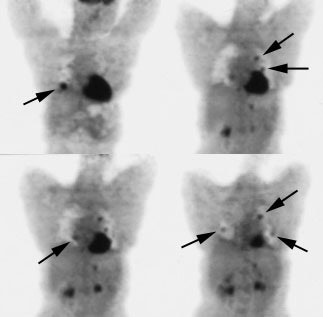
Recurrent thyroid carcinoma: The patient shown below had a history of thyroid cancer and presented with an elevated thyroglobulin level. After a negative diagnostic scan the patient received 125 mCi of I-131 and a post-therapy whole body iodine exam was performed which demonstrated some tracer uptake in the region of the thyroid bed and faint liver activity (right image below). FDG PET imaging demonstrated widespread pulmonary metastases and pathologic adenopathy in the lower left neck (black arrow). Click here to view rotating PET image (1.2MB) |
|
|
|
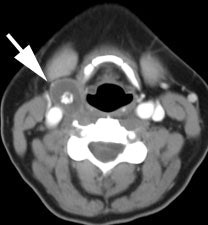
Recurrent thyroid carcinoma and a negative PET scan: The patient shown below had a history of thyroid cancer and presented with an elevated thyroglobulin level. On a diagnostic CT scan, the patient was found to have a cystic lesion in the right upper neck containing a central calcification. A whole body I-131 scan was negative and a subsequent PET scan was performed. The PET scan demonstrated no tracer uptake within the patients cystic right neck mass. At surgery, the lesion was found to be metastatic thyroid cancer. It is likely that due to the cystic nature of the lesion the PET scan did not demonstrate significant tracer uptake. |
|
|
Prognosis:
FDG imaging carries prognostic significance in patients with
recurrent thyroid cancer and is an independent predictor of
survival with a negative impact associated with a positive FDG
scan [10,33]. In fact, the volume of FDG avid disease may be the
single strongest predictor of overall survival (rather than
gender, iodine uptake, histology, or grade) [14]. Three year
survival is significantly reduced in patients with positive FDG
scans (60% survival) when compared with patients with negative
exams (98% survival) [10]. High-dose I-131 therapy generally has
little or no effect on the viability of FDG avid metastases [13].
In patients with FDG positive lesions that are not amenable to
surgical resection, thyroid hormone suppression, external beam
radiation, chemotherapy, radiofrequency ablation, and
chemoembolization can all be attempted for treatment [17].
A high SUVmax and the number of FDG avid lesions have also been
correlated with decreased overall survival [33].
68Ga-DOTATATE/DOTATOC:
In differentiated thyroid cancer, SSTR expression can be found independently of glucose transporter over expression [37]. 68Ga-DOTATATE/TOC imaging may be considered in patients with rising Tg levels and negative RAI and negative FDG imaging [37]. The agent appears to be especially promising for imaging poorly differentiated and oxyphilic subtypes of DTC [37].
Non-pathologic findings:
Following thyroidectomy, FDG uptake in the thyroid bed is a common finding and does not necessarily indicate recurrent disease unless a definite mass can be identified with other imaging modalities [2]. False positive cases have occurred in association with mediastinal tuberculous adenitis.
In Hurthle cell carcinoma:
Hurthle cell cancer of the thyroid is an uncommon follicular variant (3.6% of thyroid cancers) composed mostly of oxyphilic follicular cells and the tumor generally has a low avidity for iodine [11,14,18]. Hurthle cell is more aggressive than follicular thyroid cancer and has an overall worse prognosis [11,18]. The 20 year survival is 65% compared to 87% for papillary cancer and 81% for follicular cancer [18]. Serum thyroglobulin levels are sensitive in detecting the presence of residual or recurrent disease, but cannot localize the site of the tumor [18]. FDG accumulation is generally very intense in patients with Hurthle cell cancer and can reveal disease not detected by other imaging methods in about 50% of cases [11]. FDG PET has an overall sensitivity of 92-96%, a specificity of 80-95%, a PPV of 92%, a NPV of 80%, and an accuracy of 89-95.5% in the evaluation of Hurthle cell thyroid cancer [14,18]. False negative exams can be seen in patients with iodine avid Hurthle cell cancers [18]. The amount of FDG accumulation within the tumor may also carry prognostic significance, with a decreased 5 year survival in patients with SUV max or greater than 10 (64% survival versus 92% if SUV max was less than 10) [18].
|
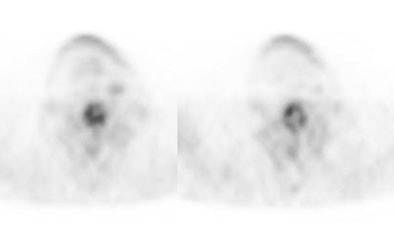
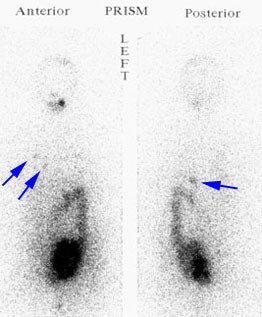
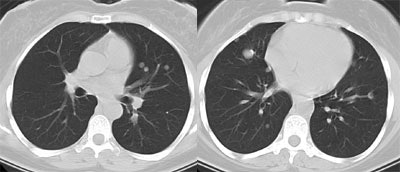
Recurrent Hurthle cell carcinoma: The patient had a history of Hurthle cell carcinoma of the thyroid. Pulmonary metastases are significantly more evident on the coincidence FDG PET examination (below left black arrows) when compared to the I131 scan (below right blue arrows). A CT scan confirmed the presence of pulmonary metastases (below). Note that a subtle bone metastasis to the high left parietal bone can be seen on I131 images. The FDG PET exam did not include this region. |
|
|
Medullary thyroid cancer:
Medullary thyroid cancer (MTC) is a relatively rare neoplasm
arising from calcitonin-secreting parafollicular cells- it
accounts for only 3-10% of all malignant thyroid cancers [9,24].
Familial forms account for about 25% of cases (MTC is associated
with multiple endocrine neoplasia tyoes 2A and 2B) [9]. Metastases
to locoregional lymph nodes is common and can be seen in 35-80% of
cases at presentation [9,24]. Distant metastases can be found in
about 20% of patients [9].
The overall 10 year survival is between 40-80% [29]. Tyrosine
kinase inhibitors, vandetanib and cabozantinib, are now being used
for the treatment of advanced MTC with favorable antitumor
activity [36]. For patients receiving vandetanib therapy, a
SUVmean of more than 4 for the most metabolically active lesion at
baseline imaging was associated with a worse prognosis (2.7 fold
shorter progression free survival) [36].
No single conventional imaging test or nuclear imaging agent has been shown to be very sensitive for the detection of metastatic MTC [9].
Primary disease: Medullary thyroid cancer usually demonstrates very positive FDG uptake and PET imaging is useful for the staging and follow-up of patients [14]. Sensitivity is 76-78% and specificity is 79% [14].
Recurrent disease: Following surgical treatment, elevation
of serum calcitonin (a specific marker for MTC) and CEA (less
specific) levels suggest persistent or recurrent disease [9]. A
short calcitonin or CEA doubling time are suggestive of
progressive disease [29].
In patients with recurrent MTC FDG PET imaging can identify more
than twice as many sites of disease than conventional imaging
modalities (CT and MR) [9]. Sensitivity for the detection of
recurrent disease is as high as 75-78% when the calcitonin level
is greater than 1000 pg/mL (and 20-37% when the level was under
1000 pg/mL) [24,33]. FDG PET imaging is less sensitive for the
detection of pulmonary and hepatic metastases compared to CT and
MR, respectively [9] and appears to be of less utility when the
calcitonin level is below 500 ng/mL [24]L [33]. The calcitonin
doubling time also affects lesion detection with shorter doubling
times correlating with higher detection rates [33]. The exam is of
much less utility when the calcitonin doubling time is greater
than 24 months [29]. Detection rates have been reported to
be 67% when the doubling time was less than 24 months, and only
26% when the doubling time was greater than 24 months [33]. CEA
levels can also affect the exams sensitivity with a detection rate
of 69% for a CEA level greater than 5 ng/mL, and 45% for a CEA
level less than or equal to 5 ng/mL [33]. CEA doubling time also
affects detection - with a detection rate of 33% for a CEA
doubling time over 24 months and 91% for patients with doubling
rates below 24 months [33].
Patients with FDG PET positive exams have a worse prognosis
compared to FDG negative patients (regardless of F-DOPA scan
findings) [29]. This is likely because aggressive
(dedifferentiated) disease has higher glucose metabolism [29].
Anaplastic thyroid cancer (ATC):
REFERENCES:
(1) J Nucl Med 1999; Chung JK, et al. Value of FDG PET in papillary thyroid carcinoma with negative I-131 whole body scan. 40: 986-992
(2) J Nucl Med 2000; Alnafisi NS, et al. FDG PET of recurrent or metastatic 131I-negative papillary thyroid carcinoma. 41: 1010-1015
(3) J Nucl Med 2000; Moog F, et al. Influence of thyroid-stimulating hormone levels on uptake of FDG in recurrent and metastatic differentiated thyroid carcinoma. 41: 1989-1995
(4) J Nucl Med 2001; Schluter B, et al. Impact of FDG PET on patientw with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131-I scan. 42: 71-76
(5) Thyroid 1994; Clark OH, et al. Managament of patients with differentiated thyroid cancer who have positive serum thyroglobulin levels and negative radioiodine scans. 4 (4): 501-505
(6) J Nucl Med 2001; Shiga T, et al. Comparison of 18F-FDG, 131I-Na, and 201-Tl in diagnosis of recurrent or metastatic thyroid cancer. 42: 414-419
(7) Head Neck 2001; Yeo JS, et al. F-18-fluorodeoxyglucose positron emission tomography as a presurgical evaluation modality for i-131 scan-negative thryoid carcinoma patients with local recurrence in cervical lymph nodes. 23: 94-103
(8) J Nucl Med 2001; Helal BO, et al. Clinical impact of 18F-FDG PET in thyroid carcinoma patients with elevated thyroglobulin levels and negative 131I scanning results after therapy. 42: 1464-1469
(9) J Nucl Med 2002; Szakall S, et al. 18F-FDG PET detection of lymph node metastases in medullary thyroid carcinoma. 43: 66-71
(10) Endocrinol Metab Clin North Am 2001; Haugen BR, Lin EC. Isotope imaging for metastaic thyroid cancer. 30: 469-492
(11) J Nucl Med 2003; Lowe VJ, et al. 18F-FDG-PET of patients with Hurthle cell carcinoma. 44: 1402-1406
(12) Eur J Nucl Med Mol Imaging 2002; Petrich T, et al. Influence of rhTSH on [(18)F] fluorodeoxyglucose uptake by differentiated thyroid carcinoma. 29: 641-647
(13) J Nucl Med 2005; Robbins RJ, et al. The evolving role of 131I for the treatment of differentiated thyroid carcinoma. 46: 28S-37S
(14) Radiol Clin N Am 2004; Zhuang H, et al. Investigation of thyroid, head, and neck cancers with PET. 42: 1101-1111
(15) J Nucl Med 2005; Mazzaferri EI. Empirically treating high serum thyroglobulin levels. 46: 1079-1088
(16) J Nucl Med 2006; Palmedo H, et al. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. 47: 616-624
(17) Thyroid 2006; Cooper DS, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. 16: 1-25
(18) J Nucl Med 2006; Pryma DA, et al. Diagnostic accuracy and prognostic value of 18F-FDG-PET in Hurthle cell thyroid cancer patients. 47: 1260-1266
(19) J Nucl Med 2006; Lubberink M, et al. Acquisition settings for PET of 124I administered simultaneously with therapeutic amounts of 131I. 47: 1375-1381
(20) J Nucl Med 2007; Shammas A, et al. 18F-FDG PET/CT in patients with suspected recurrent or metastatic well-differentiated thyroid cancer. 48: 221-226
(21)
(22) J Nucl Med 2005; Mazzaferri EI. Empirically treating high serum thyroglobulin levels. 46: 1079-1088
(23) Radiology 2007; Blodgett TM, et al. PET/CT: form and function. 242: 360-385
(24) J Nucl Med 2007; Ong SC, et al. Diagnostic accuracy of
(25) J Nucl Med 2007; Ito S, et al. Comparison of
(26) Radiology 2008; Johnson NA, Tublin ME. Postoperative surveillance of differentiated thyroid cancer: rationale, techniques, and controversies. 249: 429-444
(27) J Nucl Med 2009; Fatah-Abdul SB, et al. Identification of a
shine-through artifact in the trachea with 124I
PET/CT. 50: 909-911
(28) J Nucl Med 2012; Van Nostrand D, et al. Recombinant human
thyroid-stimulating hormone versus thyroid hormone withdrawl in
the identification of metastasis in diifferentiated thyroid cancer
with 131I planar whole-body imaging and 124I
PET. 53: 359-362
(29) J Nucl Med 2012; Verbeek HHG, et al. Clinical relevance of 18F-FDG
PET and 18F-DOPA PET in recurrent medullary thyroid
carcioma. 53: 1863-1871
(30) Radiology 2013; Nakajo M, et al. Diagnosis of metastases
from postoperative differentiated thyroid cancer: comparison
between FDG and FLT PET/CT studies. 267: 891-901
(31) J Nucl Med 2013; Lee JW, et al. Clinical utility of 18F-FDG
PET/CT concurrent with 131I therapy in
intermediate-to-high-risk patients with differentiated thyroid
cancer: dual-center experience with 286 patients. 54: 1230-1236
(32) Eur J Endocrinol 2010; Ma C, et al. The role of TSH for
18F-FDG-PET in the diagnosis of recurrence and metastases of
differentiated thyroid carcinoma with elevated thyroglobulin and
negative scan: a meta-analysis. 163: 177-183
(33) AJR 2014; Marcus C, et al. PET/CT in the management of
thyroid cancers. 202: 1316-1329
(34) J Nucl Med 2016; Beijst C, et al. Quantitative comparison of
124I PET/CT and 131I SPECT/CT
detectability. 57: 103-108
(35) J Nucl Med 2016; Kist JW, et al. 124I PET/CT to
predict the outcome of blind 131I treatment in
patients with biochemical recurrence of differentiated thyroid
cancer: results of a multicenter diagnostic cohort study
(THYROPET). 57: 701-707
(36) J Nucl Med 2018; Werner RA, et al. Predictive value of 18F-FDG
PET in patients with advanced medullary thyroid carcinoma treated
with vandetanib. 59: 756-761
(37) J Nucl Med 2020; Donohoe KJ, et al. Appropriate use criteria for nuclear medicine in the evaluation and treatment of differentiated thyroid cancer. 61: 375-396