Head and Neck Tumors:
Head and neck cancers account for about 3% to 5% of
cancers in the adult population in the US [9]. Approximately
40,000 to 50,000 new cases are diagnosed each year [31,35]. Squamous
cell carcinomas represent the majority of all malignant head and
neck tumors (more than 90% of all head and neck cancers) [15,43].
Most cases are diagnosed in patients between the ages of 50-70
years, with a 5 times higher rate in men than for women [43].
Smoking and HPV infection are among the main risk factors for
oropharyngeal squamous cell carcinoma [57], but the lesions differ
in their underlying molecular and genetic profiles [78]. HPV
associated oropharyngeal squamous cell carcinomas now account for
about 40-80% of cases [60].
The overall annual mortality ate
for head and neck cancer in the US is 23% and the 5 year survival
rate is 56% [35]. The overall survival for patients with advanced
head and neck cancer is slightly less- about 40% [9]. However,
survival varies by tumor location with cancers of the lip
associated with a 90% 5-year survival and 32% for cancer of the
hypopharynx [74]. Survival decreases with positive lymph node
involvement [74].
About 40% of patients with squamous
cell head and neck cancers have localized disease, while the
remaining 60% have advanced disease [15]. Lymph node involvement
is the most important prognostic factor affecting patient survival
[15].
HPV-positive oropharyngeal cancer:
An association between HPV infection and oropharyngeal squamous cell cancers is now recognized - especially arising from the lymphoepithelium in the tongue base and palatine and lingual tonsils [57,59,60]. Greater than 90% of HPV-positive oropharyngeal squamous cell cancers (HPV OPSCC) are associated with a single HPV type- HPV-16- which is the same sexually transmitted viral subtype responsible for cervical and anogenital malignancies [60,78]. Two HPV oncoproteins- E6 and E7 degrade p53 tumor suppressor protein and retinoblastoma protein pRB and interfere with DNA repair and apoptosis [78]. To restore cell cycle control, there is upregulation of cyclin-dependent kinase inhibitor p16 which can be detected in HPV-related tumors [78].
HPV OPSCC is characterized by a younger age (40-50 years) at onset, predominance in white men, and a strong association with sexual behavior [60,78]. Positive HPV status is associated with better response to radiation/chemotherapy, a better prognosis and overall progression-free survival (90% five year overall survival [78]) [57,59,60,74]. HPV positive lesions have higher rates of phosphatidylinositol-3'-kinase pathway alterations [74]. However, for recurrent oropharyngeal SCCa, a negative human papilloma status is associated with an overall survival advantage [68].
Despite the overall better prognosis, HPV OPSCCs are consistently poorly differentiated, demonstrate a high mitotic rate, are nonkeritonizing, and have a distinct basaloid appearance [60]. The tumors are often associated with cystic lymph node metastases and have a higher rate of nodal involvement (more advanced N-stage) than HPV-negative tumors [60]. HPB OPSCC tend to disseminate to multiple organs and unusual sites [60]. These metastases can also manifest later in the disease course- between 3-5 years after completion of treatment [60].
Non-HPV tobacco related OPSCC typically has TP53 mutations, does
not have upregulation of p16, and has a worse prognosis (40% five
year survival) [78].
Perineural involvement/Perineural spread: Perineural involvement (PNI) refers to tumor invasion into the neural space of small nerve branches confined to the tumor site [77]. Perineural spread (PNS) involves larger nerves accompanied by tumor spread along the nerves away from the primary site [77]. In head and neck mucosal squamous cell cancers, PNI confers a higher risk of local recurrence (23% vs 9%), a higher risk of metastases, and worse disease specific mortality (54% vs 25%) [77]. PNI has also been found to be a predictor of lymph node metastases [77]. Approximately, 40% of patients with PNS are asymptomatic [77]. On imaging, PNS appears as thickening, nodularity, and enhancement of the involved cranial nerve [77]. FDG PET imaging will demonstrate curvilinear increased uptake along the distribution of the involved cranial nerve [77]. In the chronic phase, there may be decreased FDG uptake in the affected musculature, which appears atrophic [77].
Accurate pre-operative TNM staging is essential for head and neck
tumors in order to plan which type of surgical neck dissection
will be used and in determining the need for post-operative
chemotherapy and radiation treatment. The effectiveness of
surgical treatment depends on the complete excision of all tumor
tissue [15]. Early stage lesions are usually treated by surgery or
radiation as a single treatment with generally good results [9,15]. More advanced cases of head and neck
cancer require chemotherapy and surgery [9,15].
With greater emphysis on organ preservation, neck dissection is
now commonly reserved for those with residual or recurrent disease
following initial therapy [66]. Therefore, evaluation of nodal
response is crucial to the adequate performance of salvage neck
dissection [66].
Locoregional and distant recurrences occur in 25-50% of patients
with advanced-stage head and neck cancer, predominantly within the
first 3 years after treatment [68].
A meticulous technique is
essential when performing head and neck FDG-PET imaging [8]. The
muscles of mastication or laryngeal muscles can mimic metastases
[14]. To avoid muscle uptake, it is important to keep the
patient in the resting state with no eating or talking during
the distribution phase following injection. Valium or Versed
should be used for muscle relaxing purposes and patients should
be appropriately monitored. The tonsils and adenoids can also
demonstrate significant tracer uptake and should not be
interpreted as pathologic [15].
Co-registered PET/CT imaging is particularly
helpful for anatomic localization of lesions in head and neck
imaging [29]. PET/CT has been suggested to be superior to PET or
CT imaging alone [3,37]. PET/CT
results in improved lesion localization, better recognition of
physiologic uptake, fewer equivocal lesions, higher radiologist
confidence, and can affect patient management in up to 18% of
cases [3,29,37]. However, involuntary
patient motion between the CT and PET exams can result in misregistration for both attenuation
correction and lesion localization [33]. Customized
head support devices, immobilizing
masks, vacuum-lock bags, and proper patient head support can be
used to minimize involuntary patient motion [8,33].
The NCCN guidelines recommend consideration of FDG
PET/CT in the assessment of the initial treatment strategy for
advanced stage III/IV oral cavity, pharynx, and larynx cancers;
nasopharyngeal cancer; head and neck cancers of unknown primary;
and mucosal melanomas [74].
Staging and primary Tumor:
Most head and neck tumors are detected on physical exam with either direct or indirect visualization [6]. Conventional imaging of head and neck cancers with CT or MR can be hampered by artifacts associated with dental metallic implants (particularly for detection of oral cavity cancers), but this does not affect PET imaging [40,45]. FDG-PET is very accurate in identifying head and neck primary tumors. In a prospective evaluation, FDG-PET correctly identified 88% of primary laryngeal tumors and 100% of malignant parotid neoplasms [16]. In another prospective study, PET correctly identified 96% of primary head and neck malignancies- only two small lesions were not identified [25]. In a prospective study of oral cavity squamous cell carcinoma, FDG PET imaging had a sensitivity of for 98.4% for the identification of the primary tumor (compared to 87% for CT and 99% for MR) [2]. In a separate study of patients with a variety of head and neck tumors, FDG PET identified 100% of the primary tumors [10]. A literature survey in the use of PET in head and neck cancer compared to CT indicates PET has a higher sensitivity (87% vs 62%) and specificity (89% vs 73%) for staging cancer [30]. In a prospective study, overall TNM stage was altered in 31% of patients on the basis of the PET scan findings [41].
PET imaging has been shown to be a useful additional to conventional staging of patients with head and neck tumors [6]. Staging by PET imaging has better prognostic properties compared to conventional imaging (PET can upstage up to 35.5% of patients and patients that are upstaged by PET have been shown to have significantly worse progression free and overall survival) [76]. PET imaging results can also impact on patient management in up to 70% of cases by confirming local disease or identifying unsuspected metastases [9]. In another study with respect to surgical planning, PET provided additional information in 31% of patients with equivocal CT or MR findings [5]. A prospective study also found that patient management plans were altered in 34% of patients on the basis of the PET exam results [41]. Other studies have shown a change in management in 18-40% of patients based upon the PET findings [41]. Preliminary data also indicate that PET exam findings can play an important role in delineating the gross tumor volume and disease extent for radiation therapy planning purposes [31].
False positive exams can occur in association with
acute inflammation (tonsillitis) [29] and benign parotid neoplasms such as Warthin?s
tumor and pleomorphic adenomas are
also positive on FDG imaging (this compromises the specificity
of the exam for evaluation of parotid lesions).
False negative exams can be seen in salivary gland
neoplasms and spindle cell neoplasms which have inherently low FDG
uptake [1]. Necrotic neoplasms may
also produce false negative exams due to insufficient
metabolically active tissue [1]. False negative exams can also
occur in areas of high physiologic activity such as pharyngeal
lymphoid tissue [74].
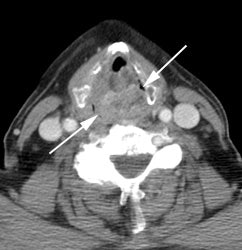
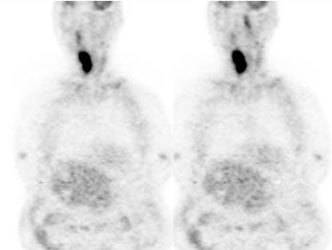
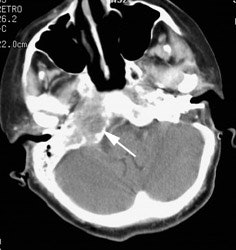
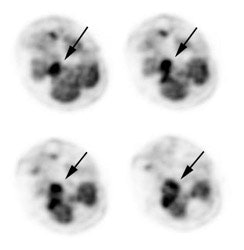
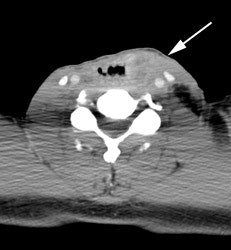
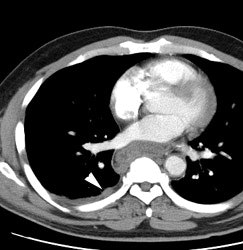
Large laryngeal cancer: The patient shown below had a large laryngeal cancer (white arrows). The PET exam demonstrated very prominent uptake within the mass, but no evidence of metastatic disease.
|
Large laryngeal cancer: The patient shown below had a large laryngeal cancer (white arrows). The PET exam demonstrated very prominent uptake within the mass, but no evidence of metastatic disease. |
|
|
Lymph nodes metastases:
In patients with squamous cell carcinoma of the head and neck, the presence of cervical lymph node metastases carries significant negative prognostic information [19,72]. The 5-year survival in the absence of nodal metastases is about 65%, while it decreases to 29% for patients with cervical nodal metastases (survival decreases by 40-50% in patients with positive nodes [74]) [19]. Between 21% to 45% of patients with clinical N0 disease and negative CT or MR exams, harbor lymph node metastases [34]. The oropharynx is generally richer in lymphatics than the oral cavity, and oropharyngeal cancers are therefore more likely to manifest with metastases to cervical lymph nodes [52]. Among patients with oral cavity SCC's, those in the retromolar trigone, floor of the mouth, and tongue show a strong predilection for lymphatic involvement [51].
Conventional imaging with CT or MRI is limited in the detection
of nodal metastases because pathologic nodes are often considered
solely on the basis of size criteria and up to 40% of metastases
occur in nodes smaller than this size [15,26].
In most studies, PET imaging has been shown to be more sensitive and specific than CT for the detection of nodal metastases [4,6,35], including contralateral nodal metastatic disease [70]. The sensitivity of FDG PET imaging for the staging of cervical lymph nodes is between 67% to 91%, and the specificity is between 80% to 100% [26,27,31,34,35,42]. Compare to a sensitivity and specificity of 65-82% and 47-85% for CT, and 80-88% and 41-79% for MR, respectively [4,31]. Overall, there is an approximately 5-10% improvement in sensitivity and specificity compared to CT or MRI [74]. For initial staging, the disease probability for PET positive lymph nodes is 81%, and 4.5% for PET negative nodes [4].
For lymph node metastases from squamous
cell carcinoma of the mouth, FDG PET has a sensitivity of 75%-
91%, and a specificity of 88%-96%- this is superior to MRI
(36%-78% and 71%-94%, respectively) [15, 16]. In a prospective
study of patients with oral cavity squamous
cell carcinoma PET had a level-by-level sensitivity for the
detection of nodal metastases of almost 75%, compared to 53% for
CT/MR (level-to-level accuracy of PET was 89.4%) [2]. The specificity of PET was 93%, compared to
94.5% for CT/MR [2]. False-positive PET exams occurred in
association with reactive and inflammatory lymph nodes [2]. In
this study, PET disclosed metastatic lesions in approximately half
of morphologically benign nodes [2]. However- small volume
metastatic disease (of about 5.5 mm of less) will generally not be
detected on PET imaging [2,34,50]-
hence- a negative PET scan does not necessarily limit the surgical
dissection [2].
In another prospective study of patients with head and neck squamous cell cancer and negative neck palpation findings (clinical N0 patients), PET/CT was superior to conventional imaging (CT/MR) for the demonstration of nodal metastases (per patient sensitivity was 71% of PET, and 50% for CT/MR; specificity was 81% and 87%, respectively) [61]. The overall accuracy was 77% for PET and 71% of CT/MR [61]. False positive results occurred due to reactive or inflammatory lymph nodes, and false negative findings were seen in associaiton with small volume disease (average sized of missed mets was 5.7 mm) [61]. In a meta-analysis, of patients with clinically negative neck examinations, PET had a sensitivity of 66% and specificity of 87% for the detection of lymph node involvement [72].
Other limitations of PET imaging is its inability to identify macroscopic extranodal spread which is associated with a 10 fold increased risk of recurrence and a 50% reduction in survival compared to nodes with no (or microscopic) extranodal spread [31]. Also- intense tumor uptake of FDG may also obscure adjacent nodes [31] and nodal necrosis may cause false-negative findings on FDG PET imaging due to the loss of viable tumor cells [2]. However, PET/CT can overcome these limitations and result in overall more accurate staging [2,31].
PET can be particularly useful
in the assessment of patients with clinical stage N0 disease
[19]. In these cases between 16%-60% of patients are
subsequently found to have occult lymph node metastases [19]. In
cases of clinical N0 disease, PET has been shown to have a
sensitivity of 78% and an accuracy of 92% (compared with a
sensitivity of 57% and an accuracy of 76% for CT) for the
detection of nodal metastases [19]. Therefore, PET can provide
additional information compare to conventional imaging regarding
the presence of lymph node metastases. However, PET imaging
cannot completely replace surgical staging of cervical nodes.
Detection of metastatic disease:
The presence of metastatic disease can significantly impact on
patient management. These patients can be spared the expense,
morbidity, and mortality associated with extensive head and neck
surgery [26]. By providing a whole body survey, FDG PET can detect
distant sites of metastatic disease and other primary neoplasms.
The overall incidence of distant metastatic disease is generally
low and quite variable (2-18%), but is higher in patients with
more advanced disease [49,74]. The most common sites for distant
metastatic disease in head and neck cancer are the lungs, bone,
and liver [49,74]. Several studies have demonstrated that PET can
detect unsuspected metastatic disease particular in patients with
advanced local-regional disease [35]. In one
study of patients with advanced stage head and neck tumors
(Stage III or IV), PET imaging identified unsuspected mediastinal nodal metastases in 17% of
patients [26]. Other authors have shown that combined
diagnostic chest CT and PET imaging has the highest sensitivity
for the detection of distant metastatic disease in high risk
patients, and that this combination does not lead to additional
cost [49]. By avoiding futile unnecessary surgery, the
pre-operative identification of metastatic disease can result in a
substantial cost savings [26]. In fact, PET imaging has been
estimated to be cost effective if metastatic disease is found in
just one of nine patients [26].
Patients with head and neck tumors also have a high incidence of
secondary tumors of the aerodigestive
tract (between
6% to 36%) [5,15,16]. FDG PET imaging can easily identify other
primary neoplasms which are routinely
missed on coventional imaging and
result in timely and appropriate treatment for these lesions
[5,75].
|
|
|
|
|
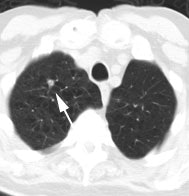
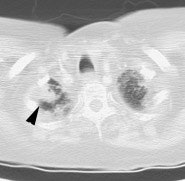
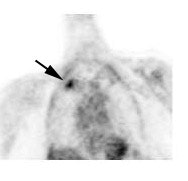
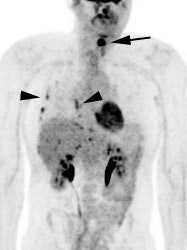
Metastatic head and neck cancer with secondary lung malignancy: The patient shown below had a history of head and neck cancer. The PET scan demonstrated a metastatic lesion to the right temporal bone. Also noted was intense uptake in the right lung apex(black arrow below right)- on CT this was felt to represent scarring (black arrowhead). FDG uptake is highly suggestive of a separate primary lung cancer. |
|
|
Unknown primary:
Between 2-9% of patients with squamous cell carcinoma of the head and
neck present with metastatic cervical lymph nodes without an
identifiable primary site by clinical exam [17,31,35]. A dedicated clinical exam and
conventional CT/MR imaging will identify the site of the primary
tumor in the majority of patients; however, between 32%-45% of
patients will have still an unknown primary lesion [17]. In this
subset of patients, PET imaging has been shown to be superior to
convention CT/MR imaging and can provide
additional useful information by identifying the primary site of
the tumor in about 25% to 69% of patients [6,29,31,35,41,67] and
in revealing additional unsuspected sites of disease [15,17,21].
False negative results can occur, particularly in association
with small primary tumors (less than 10 mm) [67].
Treatment Planning:
Using PET/CT data improves target volume delination when compare
to CT or MR imaging alone [55]. The PET exam findings can have
significant influence on CT or MR based tumor contours by
incorporating biologic/metabolic features of the tumor and thereby
reducing the risk of geometric misses and minimizing dose to
normal tissue [55]. Treatment plan can be changed in up to 15% of
patients when PET data is incorporated into planning [55]
Response to therapy:
Between 50-70% of patients with head and
neck squamous cell carcinoma achieve a complete response, while
between 30-50% will have residual or recurrent disease [59].
Unsuspected residual disease can be found in up to 19.5% of
patients [65]. Neoadjuvant chemotherapy or combined chemoradiotherapy is used to decrease
tumor size before surgery in locally advanced-stage head and neck
cancer [9]. Neoadjuvant therapy has
been shown to improve rates of clinical complete response at the
primary site, improve locoregional
control, and improve survival [39]. A cure in locally advanced
head and neck cancer is associated with complete remission
following first-line therapy [22]. Neither CT or MR can reliably
differentiate post-treatment change from residual tumor [9,10]. FDG-PET imaging can be used to
monitor response to therapy and identify residual viable tumor
when it is otherwise unsuspected [9,10,28].
Patients that have a favorable response to therapy
generally demonstrate a significant reduction in FDG uptake from
baseline values (mean reduction 82%) [27]. Positive results on
intratherapy or post therapy scans are strongly predictive of an
increased risk for progression, recurrence, and death,
particularly within 2 years, but also for up to 5 years [69].
Early recognition of resistance to chemotherapy can result in
prompt institution of secondary treatment strategies [9].
Tumor response can be assessed quantitatively or qualitatively [74]. The PRECIST criteria suggest a reduction of at least 30% in SUVmax is required to document a partial tumor response [74]. The EORTC criteria suggest a decrease in SUVmax of between 15-25% is indicative of a good treatment response [74]. Qualitative assessment can be performed to accurately assess lesion response using the Hopkins criteria which compares lesion FDG avidity to the liver and internal jugular vein [74,79].
Hopkins Criteria Five Point Post Therapy Assessment Scoring
System:
| Score |
FDG Uptake Pattern |
Response |
| 1 |
Uptake in lesion and nodes less than
IJV |
Complete metabolic response |
| 2 |
Focal uptake above IJV, but less than
liver |
Likely complete response |
| 3 |
Diffuse uptake at primary site or nodes
greater than IJV or liver |
Likely post XRT inflammation |
| 4 |
Focal uptake at primary or nodes above
liver |
Likely residual tumor |
| 5 |
Focal and intense uptake at primary or
in nodes |
Positive residual tumor |
Scores of 1, 2, or 3 are determined to be negative for residual disease, whereas a score of 4 or 5 are positive [79].
Results: Tumors that respond to treatment have decreased
metabolic activity and hence, decreased FDG uptake [10,18,25]. The overall sensitivity of PET for
residual cancer after therapy is between 68-100% [23,25,39,52,65,71].
Qualitative assessment using a 5 point scale (or Hopkins
criteria) has also been shown to be a sensitive and accurate
method for exam interpretation [65,73] with a high specificity
(91%) and high NPV (92%) [79]. Additionally, results based on
Hopkins criteria analysis have been shown to alter patient
management in about 64% of patients [79]. Qualitative results can
be used to predict survival outcomes in patients with residual
neck nodes post therapy better than conventional imaging [73].
Patients with residual cervical nodes that are negative by Hopkins
criteria have been shown to have 7 times greater overall survival
compared to those with PET positive nodes, and are 6 times less
likely to have progression of disease [73].
When to scan: The timing of post-therapy PET imaging is important
for an accurate assessment of response to treatment. The issue of
when to scan is complex and there is conflicting data regarding
this issue. PET imaging performed too soon after treatment may
produce both false-positive and false-negative results [52]. Monitoring response to radiation therapy can be complex
due to inflammatory and tissue healing responses [9]. Some
authors advocate waiting 3 to 4 months following completion of
radiation therapy prior to PET imaging to assess for residual or
recurrent [6,16,24,25,52,59]. Post-treatment scans
performed earlier than this time may demonstrate decreased FDG
uptake even in the presence of residual disease (ie: false-negative results) [16,25]. This may be related to altered FDG uptake kinetics related to
derangements in cellular glucose transport or vascular damage
(that hampers tracer accumulation [52]) rather than actual cell
death [9] and residual microscopic disease would also go
undetected. False positive FDG uptake within
non-pathologic lymph nodes can be seen following radiation (and
chemo) therapy possibly secondary to an inflammatory reactive
change [10,29]. These nodes can usually
be correctly identified as they generally do not demonstrate
increased tracer uptake on the pre-therapy baseline exams [10]. In general, a waiting period of about 10-12 weeks
following therapy completion is recommended, unless clinical
management requires it at an earlier time [39].
However, early PET FDG imaging during therapy has
been studied [22,27,56]. FDG PET
exams performed early (at around 20 Gy) or 1 to 3 weeks
following initiation of therapy can be used to predict treatment
response, local control and survival [22,56] and persistent
tumor uptake one month following radiation therapy is strongly
suggestive of residual disease [18]. Authors have
found that imaging as early as one month following completion of
radiation therapy can be performed with excellent sensitivity
(88%) and specificity (95%) [36]. The better prognotic value of
early imaging may be partly explained by the inability of PET
scans obtained later udring the course of therapy to identify
miscroscopic residual disease that is ultimately responsible for
tumor recurrence [56]. Also- post radiation inflammatory changes
can also confound later PET imaging [56].
A waiting period of 5 to 7 days following needle biopsy or 6 weeks after surgical resection is recommended to avoid false-positive findings associated with inflammation [27]. Osteoradiation necrosis of the mandible and thyroid cartilage can also demonstrate increased FDG uptake in patients that have received radiation therapy.
Prognosis:
For non-surgical therapy in head and neck cancer
patients it is difficult to predict treatment outcomes reliably
even in patients within the same TN category [20]. The amount of
FDG uptake (as measured by SUVmax) has been shown to correlate
with predicting local control and disease free survival in
patients with head and neck cancers [9,20,38,41,63]. Generally,
the higher the SUV the lower the disease free survival rate [9,38,63], however, this has not been
confirmed in all studies [25]. None-the-less, high FDG uptake
(SUVmax greater than 5.5/or other authors suggest 10 [63]) may
help to identify a subgroup of patients that will require more
aggressive treatment protocols [20]. In a study of patients with
squamous cell carcinoma of the oropharynx, high pre-treatment SUV
values were an independent prognostic indicator and could also
be used to guide more effective treatment strategies [38].
Another factor associated with overall worse prognosis is the
presence of tumor metabolic heterogeneity on PET imaging [57].
For patients with non-squamous cell carcincoma of
the head and neck, higher FDG uptake is also associated with
decreased overall survival [54]. Another prospective study found
that patients who had additional lesions identified on PET that
were not seen on conventional imaging, were less likely to
achieve a complete response and had a trend toward poorer
disease free survival [41].
Other PET findings associated with decreased
disease free and overall survival include the metabolic tumor
burden/volume (> 20 cm3 - the mean tumor volume
can be measured by contouring margins defined by thresholds,
total lesion glycolysis (> 70 g - the TLG is calculated by
multiplying MTV by the mean SUV), and a ring-shaped tumor uptake
pattern (suggestive of central necrosis) [63,64]. Other authors suggest that a metabolic tumor volume
greater than 41 mL is associated with a 2.4 fold higher
recurrence or death rate [73]. The presence of lymph node
necrosis or lymph node and 40% of the maximum nodal total lesion
glycolysis of 38 g or greater are also poor prognostic features
[66].
Following therapy, a positive PET scan has been
found to be associated with a greater than 6-fold increase in
the risk of death within 2 years [74].
Recurrent disease:
Locoregional recurrence of advanced head and neck squamous cell cancer (SCC) can occur in up to 45% of patients [46]. The risk for recurrence is highest for Stages III and IV (compared to stages I and II) [46]. Most local recurrences are seen within the first 2 years following radiation therapy [24,46,59]. Distant metastases are seen less often (5-10% of patients, other authors indicated 20-30% [52]) and are more commonly seen with oropharyngeal SSC (compared to SCC of the oral cavity) [46].
Early diagnosis of local recurrence is important for the prompt institution of salvage therapy [24,42]. A delay in the detection of recurrence has been shown to be associated with a deleterious clinical outcome [42]. Only about 20% of patients with recurrent disease survive at one year [9]. However, patients with recurrent, early stage disease who undergo salvage surgery have a 70% 2-year relapse free survival [42].
One of the most useful applications of PET imaging is the detection of residual or recurrent head and neck neoplasm [1]. Following treatment, there is distortion of the normal anatomy and it can be very difficult to distinguish post-therapeutic change from recurrent or residual tumor with conventional imaging modalities [6,32,42]. Even on laryngoscopy, it can be difficult to distinguish post-radiation change and edema from recurrence [24] and biopsy in this setting is also not without potential complications [24]. FDG PET imaging can be used to aid in identifying a subgroup of patients that should proceed to biopsy [24].
PET has been shown to have a sensitivity between 88% to 100% for
the detection of recurrent cancer (specificity 60-100%
[1,32,42,46]). A meta-analysis concluded FDG PET had 94%
sensitivity, 82% specificity, 75% PPV, and 95% MPV in detecting
residual or recurrent HNSCC [59]. Because the positive prdictive
value of a positive scan is low (75%), a positive scan warrants
further evaluation with biopsy [24,27,59]. Even if the biopsy
result proves to be negative, patients with a positive PET scan
should undergo close follow-up as some of these patients will
subsequently be shown to actually have recurrence [24].
Compared to regular follow-up, the use of surveillance PET can
result in a change in diagnostic procedures or patient treatment
in up to 63% of patients [46]. When used for surveillance for
tumor recurrence, the best time to perform PET imaging is between
3-6 months after completion of treatment in order to decrease the
risk of false positive exams [46].
One point to remember is that a positive scan is not always due to malignancy (the specificity of a positive scan can be as low as 63% [24]). Infection, reactive lymph nodes, radiation necrosis, and dysplasia can all result in increased FDG uptake [24,42]. False negative exams can occur if a patient is scanned too soon after completion of chemo/radiaiton therapy [32]. A minimum of 3 to 4 months following completion of radiation therapy is recommended prior to PET imaging to minimize the chance of obtaining a false positive or a false negative exam [6,16,24,25,32].
Patients with a negative PET scan are at a very low risk for having recurrence and follow-up of these patients can be performed (ie: the exam has a very high negative predictive value) [24,42].
Another important role for FDG-PET imaging is in the evaluation
of residual soft tissue abnormality following radiation therapy.
The PET exam can very accurately distinguish residual tumor from
scar with an accuracy of 81% (compared to 42% for the CT/MR exam)
[16].
|
Recurrent head and neck cancer: The patient shown in the case below had a history of squamous cell carcinoma of the head and neck. The PET scan demonstrated focal tracer uptake in the left lower neck consistent with recurrent disease (black arrow on PET scan ,white arrow on CT). The PET scan also detected scattered foci of increased tracer activity within the right pleural space which corresponded to metastatic tumor implants (black arrow head on PET scan, white arrowhead on CT). |
|
|
|
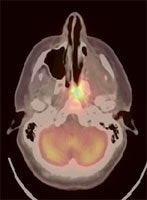
Recurrent head and neck cancer: The case below is from a 23 year old female with moderately differentiated squamous cell carcinoma of the left ethmoid sinus. Following pre-operative chemo and radiation therapy, residual tumor was surgically removed. Four months later a PET/CT exam showed local recurrence in the posterior nasal cavity and was also positive for cervical lymphadenopathy (not shown). The patient subsequently underwent a modified radical neck dissection, nasal endoscopy, and endoscopic tumor excision. Fusion images such as these shown below provide the greatest degree of anatomic correlation with the metabolic information derived from the FDG PET exam. Case courtesy University of Pittsburgh and CTI PET Systems, Inc. |
|
|
Other agents for head and neck cancer imaging:
REFERENCES:
(1) Radiographics 2005; Fukui MB, et
al. Combined PET-CT in the head and neck. Part
2. Diagnostic uses and pitfalls of
oncologic imaging. 25: 913-930
(2) J Nucl Med 2005; Ng SH, et al. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcioma: a prospective study of 124 patients with histologic correlation. 46: 1136-1143
(3) Radiology 2005; Branstetter BF, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone. 235: 580-586
(4) Radiol Clin N Am 2004; Zhuang H, et al. Investigation of thyroid, head, and neck cancers with PET. 42: 1101-1111
(5) AJR 2005; Dammann F, et al. Rational diagnosis of squamous cell carcinoma of the head and neck region: comparative evaluation of CT, MRI, and 18F-FDG PET. 184: 1326-1331
(6) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(7) Radiology 2004; Schoder H, et al. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. 231: 65-72
(8) J Nucl Med 2004; Vogel WV, et al. PET/CT: panacea, redundancy, or something in between. 45 (Suppl): 15S-24S
(9) J Nucl Med 2004; Kostakoglu L, Goldsmith SJ. PET in the assessment of therapy response in patients with carcinoma of the head and neck and of the esophagus. 45: 56-68
(10) J Nucl Med 2003; Kitagawa Y, et al. Prospective comparions of 18F-FDG PET with conventional imaging modalities (MRI, CT, and 67Ga scintigraphy) in assessment of combined intraarterial chemotherapy and radiotherapy for head and neck carcinoma. 44: 198-206
(11) J Nucl Med 1995; Braams JW, et al. Detection of lymph node metastases of squamous-cell cancer of the head and neck with FDG-PET and MRI. 36: 211-16
(12) Radiology 1995; Oct.: p.205
(13) J Nucl Med 1995; Laubenbacher C, et al. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI, and endoscopy for staging head and neck squamous-cell carcinomas. 36: 1747-57
(14) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(15) J Cancer Res Clin Oncol 2000; Ilknur AK, et al. Positron emission tomography with 2-[18F] fluoro-2-deoxy-D-glucose in oncology: Part II: The clinical value in detecting and staging primary tumours. 126: 560-574
(16) Head Neck 1998; McGuirt WF, et al. PET scanning in head and neck oncology: A review. 20: 208-15
(17) Head Neck 1998; Mendenhall WM, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. 20: 739-44
(18) Radiol Clin N Am 2001; Delbeke D, Martin WH. Positron emission tomography in oncology. 39: 883-917
(19) Laryngoscope 1998; Myers LL, et al. Positron emission tomography in the evaluation of the N0 neck. 108: 232-236
(20) J Clin Oncol 2002; Allal AS, et al. Standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. 20: 1398-1404
(21) Head Neck 2003; Fogarty GB, et al. The usefulness of fluorine 18-labelled deoxyglucose positron emission
tomography in the investigation of patients with cervical lymphadenopathy from an unknown
primary tumor. 25(2):138-145
(22) Head Neck 2002; Brun E, et al.
FDG PET studies during treatment: prediction of
therapy outcome in head and neck squamous
cell carcinoma. 24:127-135
(23) Head Neck 1997; Lowe VJ, et al. Evaluation of chemotherapy
response in patients with advanced head and neck cancer using
[F-18] fluorodeoxyglucose positron
emission tomography. 19: 666-674
(24) Head Neck 2001; Terhaard CH, et
al. F-18-fluoro-deoxy-glucose positron-emission tomography
scanning in detection of local recurrence after radiotherapy for
laryngeal/pharyngeal cancer. 23: 933-941
(25) Head Neck 2001; Greven KM, et
al. Serial positron emission tomography scans following radiation
therapy of patients with head and neck cancer. 23: 942-946
(26) Head Neck 2001; Teknos TN, et
al. Positron emission tomography in the evaluation of stage III
and IV head and neck cancer. 23: 1056-1060
(28) AJR 2003; Damascelli B, et al. A novel intraarterial chemotherapy using paclitaxel in albumin nanoparticles to treat advanced squamous cell carcinoma of the tongue: preliminary findings. 181: 253-260
(29) Radiology 2004; Schoder H, et al. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. 231: 65-72
(30) J Nucl Med 2004; Scarfone C, et al. Prospective feasibility trial of radiotherapy target definition for head and neck cancer using 3-dimensional PET and CT imaging. 45: 543-552
(31) AJR 2005; Kapoor V, et al. Role of 18F FDG PET/CT in the treatment of head and neck cancers: principles, technique, normal distribution, and initial staging. 184: 579-587
(32) AJR 2005; Kapoor V, et al .
(33) J Nucl Med 2005; Beyer T, et al. On the use of positioning aids to reduce misregistration in the head and neck in whole-body PET/CT studies. 46: 596-602
(34) J Nucl Med 2006; Schoder H, et al.
(35) J Nucl Med 2007; Quon A, et al. Clinical role of
(36) J Nucl Med 2007; Kim SY, et al.
The feasibility of
(37) Radiology 2007; Blodgett TM, et al. PET/CT: form and function. 242: 360-385
(38) J Nucl Med 2007; Kim SY, et al. Use of 18F-FDG PET for primary treatment strategy in patients with squamous cell carcinoma of the oropharynx. 48: 752-757
(39) J Nucl Med 2008; Ong SC, et al. Clinical utility of
(40) J Nucl Med 2008; Baek CH, et al. Tumor volume assessment by 18F-FDG PET/CT in patients with oral cavity cancer with dental artifacts on CT or MR images. 49: 1422-1428
(41) J Nucl Med 2008; Scott AM, et al. PET changes management and improves prognostic stratification in patients with head and neck cancer: results of a multicenter prospective study. 49: 1593-1600
(42) J Nucl Med 2009; Abgral R, et al. Does
(43) J Nucl Med 2009; Nothelfer EM, et al. Identification and characterization of a peptide with affinity to head and neck cancer. 50: 426-434
(44) J Nucl Med 2009; Dirix P, et al. Dose painting in
radiotherapy for head and neck squamous
cell carcinoma: value of repeated functional imaging with
(45) J Nucl Med 2009; Rodrigues RS, et al. Comparison of whole-body PET/CT, dedicated high-resolution head and neck PET/CT, and contrast-enhanced CT in preoperative staging of clinically M0 squamous cell carcinoma of the head and neck. 50: 1205-1213
(46) J Nucl Med 2009; Krabbe CA, et al.
(47) J Nucl Med 2010; Troost EGC, et al. Innovations in radiotherapy planning of head and neck cancers: role of PET. 51: 66-76
(48) J Nucl Med 2010; Wang W, et al. Pharmacokinetic analysis of hypoxia 18F-fluoromisonidazole dynamic PET in head and neck cancer. 51: 37-45
(49) J Nucl Med 2010; Uyl-de Groot
CA, et al. Chest CT and whole-body
(50) J Nucl Med 2011; Liao CT, et al. PET and PET/CT of the neck lymph nodes improves risk prediction in patients with squamous cell carcinoma of the oral cavity. 52: 180-187
(51) Radiographics 2011; Trotta BM, et al. Oral cavity and oropharyngeal squamous cell cancer: key imaging findings for staging and treatment planning. 31: 339-354
(52) Radiographics 2011; King KG, et al. Cancers of the oral cavity and oropharynx: FDG PET with contrast-enhanced CT in the posttreatment setting. 31: 355-373
(53) Radiographics 2011; Nayak S. Invited commentary- cancers of
the oral cavity and oropharynx: FDG
PET with contrast-enhanced CT in the posttreatment
setting. 31: 374-376
(54) AJR 2011; Imsande HM, et al. Use of 18F-FDG
PET/CT as a predictive biomarker of outcome in patients with
head-and-neck non-squamous cell carcinoma. 197: 976-980
(55) Radiographics 2012; Kovalchuk N, et al. Deformable
registration of preoperative PET/CT with post operative radiation
therapy planning in head and enck cancer. 32: 1329-1341
(56) J Nucl med 2013; Menda Y, Buatti JM. PET imaging during
radiotherapy of head and neck cancer. 54: 497-498
(57) J Nucl Med 2013; Cheng NM, et al. Textural features of
pretreatment 18F-FDG PET/CT images: prognostic
significance in patients with advanced T-stage oropharyngeal
squamous cell carcinoma. 54: 1703-1709
(58) Radiographics 2013; Bhatnagar P, et al. Functional imaging
for radiation planning, response assessment, and adaptive therapy
in head and neck cancer. 33: 1909-1929
(59) J Nucl Med 2013; Paidpally V, et al. Addition of 18F-FDG
PET/CTto clinical assessment predicts overall survival in HNSCC: a
retrospective analysis with followu-up for 12 years. 54: 2039-2045
(60) J Nucl Med 2014; Subramaniam RM, et al. PET/CT imaging and
human papilloma virus-positive oropharyngeal squamous cancer:
evolving clinical imaging paradigm. 55: 431-438
(61) Radiology 2014; Roh JL, et al. 18F
fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma
with negative neck palpation findings: a prospective study. 271:
153-161
(62) J Nucl Med 2014; Kuhn FP, et al. Contrast-enhanced PET/MR
imaging versus contrast-enhanced PET/CT in head and neck cancer:
how much MR information is needed? 55: 551-558
(63) AJR 2014; Koyasu S, et al. Prognostic value of pretreatment
18F-FDG PET/CT parameters including visual evaluation
in patients with head and neck squamous cell carcinoma. 202:
851-858
(64) J Nucl Med 2014; Pak K, et al. Prognostic value of metabolic
tumor volume and total lesion glycolysis in head and neck cancer:
a systematic review and meta-analysis. 55: 884-890
(65) J Nucl Med 2014; Marcus C, et al. Head and neck PET/CT:
Therapy response interpretation criteria (Hopkins criteria) -
interreader reliability, accuracy, and survival outcomes. 55:
1141-1416
(66) J Nucl Med 2015; Lin YC, et al. Risk stratification of
metastatic neck nodes by CT and PET in patients with head and neck
cancer receiving definitive radiotherapy. 56: 183-189
(67) Radiology 2015; Lee JR, et al. Detection of occult primary
tumors in patients with cervical metastases of unknown primary
tumors: comparison of 18F-FDG PET/CTwith
contrast-enhanced CT or CT/MR imaging- prospective study. 274:
764-771
(68) AJR 2015; Sheikhbahaei S, et al. Diagnostic accuracy of
follow-up FDG PET or PET/CT in patients with head and neck cancer
after definitive treatment: a systematic review and meta-analysis.
629-639
(69) AJR 2015; Sheikhbahaei S, et al. Intratherapy or posttherapy
FDG PET or FDG PET/CT for patients with head and neck cancer: a
systematic review and meta-analysis of prognostic studies. 205:
1102-1113
(70) Radiology 2016; Park JT, et al. 18F-FDG PET/CT
versus CT/MR imaging and the prognostic value of contralateral
neck metastases in patients with head and neck squamous cell
carcinoma. 279: 481-491
(71) AJR 2016; Taghipour M, et al. FDG PET/CT in patients with
head and neck squamous cell carcinoma after primary surgical
resection with or without chemoradiation therapy. 206: 1093-1100
(72) AJR 2016; Kim SG, et al. Potential role of PET/MRI for
imaging metastatic lymph nodes in head and neck cancer. 207:
248-256
(73) AJR 2016; Wray R, et al. Therapy response assessment and
patient outcomes in head and neck squamous cell carcinoma: FDG PET
Hopkins criteria versus residual neck node size and morphologic
features. 207: 641-647
(74) AJR 2017; Goel R, et al. Clinical practice in PET/CT for the
management of head and neck squamous cell cancer. 209: 289-303
(75) J Nucl Med 2017; Rohde M, et al. Head-to-head comparison of
chest x-ray/head and neck MRI, chest CT/head and neck MRI, and 18F-FDG
PET/CT for detection of distant metastases and synchronous cancer
in oral, pharyngeal, and laryngeal cancer. 58: 1919-1924
(76) J Nucl Med 2018; Rohde M, et al. A PET/CT-based strategy is
a stronger predictor of survival than a standard imaging strategy
in patients with head and neck squamous cell carcinoma. 59:
575-581
(77) J Nucl Med 2019; Lee H, et al. An imager's guide to
perineural tumor spread in head and neck cancers: radiologic
footprints on 18F-FDG PET, with CT and MR correlates.
60: 304-311
(78) Radiographics 2019; Parvathaneni U, et al. Advances in
diagnosis and multi-disciplinary management of oropharyngeal
squamous cell carcinoma: state of the art. 39: 2055-2068
(79) AJR 2020; Banks KP, et al. It's about quality, not quantity: qualitative FDG PET/CT criteria for therapy response assessment in clinical practice. 215: 313-324