I-131 Ablation for Thyroid Neoplasms
General Principles
I-131 has a physical half-life of 8.05 days. It decays by high energy gamma photon (364 keV) and particulate emissions (beta particles). The beta emission has an average energy of 192 keV (max energy = 607 keV) and the beta particle will deposit the majority of its energy within 2.2 mm of its site of origin [34]. After oral administration, only approximately 20% of the blood iodine is absorbed by the thyroid tissue; the remainder is largely cleared through the urine (up to 75%) [128]. Because it is primarily concentrated in thyroid tissue, I-131 can be used in the treatment of thyroid cancer. Although iodine metabolism in thyroid cancer is altered with decreased iodine uptake, markedly reduced iodine organification, and a reduced half-life compared to normal thyroid tissue; thyroid tumors usually continue to express TSH receptors and will increase iodine uptake under TSH stimulation [57].
In order to ablate the thyroid bed in post surgical patients, a dose of 30,000 rads (300 Gy) is needed to be delivered to the remaining thyroid tissue [106]. Two important determinants of the success of thyroid ablation are the mass of remaining thyroid tissue in the neck, and the initial dose rate to this tissue. Dose rates below 300 rad/hr and/or more than 5 gm of residual thyroid tissue are associated with a lower success rate for complete ablation [2]. A dose of at least 7000 rads is desired at sites of residual disease.
Nodal metastases require a dose of at least 8500 rads (85 Gy- this dose is associated with a high complete lesion response rate of 80-90% [106]), with little effect shown when the delivered dose is below 3500 rads [11]. In general, I131 is less effective in bulky disease with a diameter greater than 1-2 cm and surgical excision before radioiodine may yield better results [99]. Other authors indicate that lymph node and lung metastases which receive more than 80-100 Gy are likely to achieve a complete response [122]. Lesions reaching 20-80 Gy will probably partially respond, and lesions that reach less than 20 Gy will probably not respond [122]. An important consequence is that sublethal dosing may lead to the survival of more radio-iodine resistant tumor cell clones and reduce the effect of subsequent therapies [122].
For bone metastases, an absorbed dose threshold of 85 Gy may be too low to achieve high therapeutic efficacy and unfortunately, bone metastases often become resistant after initial radioiodine therapy [107]. An absorbed dose threshold range of 350-650 Gy has been shown to achieve a response rate between 70-80% for bone metastases (4-5 times higher than for lymph node metastases) [107]. Large bone metastases with non-uniform tracer uptake on 124I PET are the most difficult to treat effectively, unless the average absorbed dose is considerably higher [107]. In patients with metastatic disease, the ability of the tumor to concentrate radioiodine affects prognosis with a 10-year survival rate of approximately 60% in radioiodine avid lesions versus 10% in refractory tumors [112].
Although it is generally recommended to limit treatment to yearly intervals, if necessary, therapy for metastases may be repeated as necessary every 3 to 6 months for up to 5 to 10 treatments.
Indications
Near-total thyroidectomy spares the posterior capsule on the side contralateral to the carcinoma in an attempt to preserve parathyroid tissue. The goal of RAIT treatment should be goal oriented and characterized as remnant ablation, adjuvant treatment, or treatment of known disease [127]:
Thryoid remnant ablation: To destroy the small amount of
thyroid tissue remaining in the neck after surgery for
facilitating long term followup (optimize serum Tg
interpretation and improve the quality of future I131 imaging)
[127,131].
For adjuvant therapy- adjuvant therapy is given based on risk, rather than for demonstrable disease [119]. These patients have factors that place them at high risk of having residual microscopic foci of thyroid cancer tissue following surgical resection or who are at high risk of developing recurrent disease [120,127]. The objective is to minimize risk of disease recurrence while improving disease-specific survival [127,132]. It is accepted that a proportion of patients who receive adjuvant treatment will have already been cured by their primary surgery [119]. In intermediate risk patients, adjuvant I131 has been shown to be associated with improved overall survival [132].
For the treatment of functional metastases- about 10% of patients with differentiated thyroid cancer have distant metastases at presentation or develop distant metastases during followup [122]. Treatment aims to eliminate iodine-avid metastatic disease, improve progression-free, disease-specific, and overall survival [127,131]. The National Thyroid Cancer Therapy Cooperative Study Group reported improved overall survival and disease-specific survival in patients with advanced tumors and regional and/or distant metastatic disease who received post-operative I131 therapy [132]. That same group has also demonstrated improved disease-free survival for stage II patients that receive I131 therapy [132]. If the metastases can be completely cured by radioiodine therapy, the overall 10 year survival is 92%, compared with 29% in patients with residual disease [122]. It is also important to note that for patients with distant metastases, a delay of > 6 months in administration of I131 therapy is associated with decreased survival [132].
For the treatment of recurrent thyroid cancer-
For the treatment of patients with elevated thyroglobulin
levels, but a negative I-131 scan (suspected, but unproven
metastatic disease) [3,33,98].
Despite the previous employment of I-131 ablation following thyroidectomy in most patients, this approach has been challenged by evidence that remnant ablation does not improve disease-specific survival in low-risk patients [98,99]. The American Thyroid Association Practice Guidelines for the management of patients with well-differentiated thyroid cancer following thyroidectomy indicate that post operative management should be tailored based upon individual assessment including tumor stage and risk of recurrence assessment/risk stratification [91,98,119].
Patients are considered low risk if they have unifocal or multifocal tumors smaller than 1 cm (microcarciomas), had complete resection of all tumor, no aggressive tumor histology, no invasion of locoregional tissues, no vascular invasion, and no metastases [91,98]. Patients with microscopic invasion into the perithyroid tissues, cervical lymph node metastases, aggressive histology, or vascular invasion are classified as being at intermediate risk [91]. Patients with macroscopic gross extrathyroid extension, incomplete tumor resection, or distant metastases are classified as being at high risk for recurrence [91].
The American Thyroid Association Guidelines Task Force stratifies
the risk of tumor recurrence [123]:
1- Low risk: absence of local or distant metastatic disease, absence of extracapsular or vascular invasion by the primary tumor, complete surgical resection, non-aggressive histologic characteristics, and negative I-131 whole body imaging
2- Intermediate risk: microscopic invasion of peri-thyroid soft tissues, aggressive histologic features (tall cell, insular, columnar subtypes), or microvascular invasion
3- High risk: macroscopic extracapsular tumor invasion, incomplete tumor resection, or distant metastases. Recurrence is most commonly seen in the anterior cervical lymph nodes or thyroid bed and most commonly occurs within the 1st decade after treatment.
Low risk patients may not require I131 ablation therapy [91,98]. However, radioiodine therapy has been shown to significantly improve survival in low risk patients with PTC diameters of 1.0-2.0 cm and 2.1-4.0 cm, and omission of RAI is associated with an increased disease specific mortality [120]. The American Thyroid Association guidelines recommend I-131 ablation for patients with high risk features of early disease including multifocal stage I disease, cervical lymph node or distant metastases, evidence of microvascular invasion, tumors larger than 4 cm (or 1 to 4 cm confined to the thyroid that have documented nodal metastases or other high risk features), and more aggressive tumor histologic features [80,98]. In patients with pT3N0M0 or pT1-pT3N1M0 disease, RAI has been shown to reduce mortality by 29%, compared to patients that did not receive treatment [120]. The proportion of patients in clinical remission (no evidence of disease) after total thyroidectomy and RAIT is 80-90% in patients with low risk, approximately 60% in those with intermediate risk, and less than 30% in patients with high risk [127].
The Society of Nuclear Medicine recommends I131 treatment should be considered in the post surgical management of patients with a maximum tumor diameter greater than 1cm, or with a diameter less than 1 cm in the presence of high-risk features such as aggressive histology (Hurthle cell, insular, diffuse sclerosing, tall cell, columnar cell, trabecular, solid, and poorly differentiated subtypes of papillary carcinoma), lymphatic or vascular invasion, lymph node or distant metastases, multifocal disease, capsular invasion or penetration, perithyroidal soft-tissue involvement, or an elevated anti-thyroglobulin antibody level after thyroidectomy (so that scintigraphy can be used for surveillance) [99].
The American Thyroid Association guidelines recommend against I131 ablation in patients with unifocal or multifocal tumors smaller than 1 cm (microcarcinomas) without high risk features [102]. However, even patients with microcarcinomas are at risk for cancer recurrence [121]. In one study, metastases occurred in 8.5% of previously ablated patients with microcaricnoma, including patients considered at very low risk [121]. The presence of minimal extrathyroidal extension, even in patients with microcarcinomas, has been shown to be associated with an increased risk for lymph node metstases at the time of thyroidectomy, as well as an increased risk for recurrent disease with lymph node or distant metastases [130].
Thyroid stimulating hormone (TSH) suppressive therapy is also known to delay or slow progression in metastatic thyroid cancer, resulting in improved overall survival as compared with patients without TSH suppression [122]. However, long-term TSH suppression may increased the incidence or severity of cardiac arrhythmias and reduce bone density [122]. The dose of levothyroxine should be adjusted to obtain a TSH value of 0.1 mU/L or slightly below, since further suppression to an undetectable level has not been shown to improve clinical outcomes [122].
Absolute Contraindications to I-131 Therapy
Pregnancy: Radioiodine freely crosses the placenta. The fetal thyroid extracts/concentrates iodine after the 10-12th week and the radiation will destroy the thyroid gland and result in severe hypothyroidism. Additionally, activity in the maternal bladder causes significant fetal irradiation. It is recommended that conception be delayed for 1 year after high-dose I-131 therapy to permit adequate control of thyroid hormone status [57]. Even 123I can cause substantial damage to fetal thyroid tissue at the administered doses for whole body imaging (2-3 mCi) [126].
Breast feeding: Both iodine and pertechnetate are excreted in breast milk. Complete cessation of breast feeding is required following I-131 administration [126].
Elevated iodine levels:
Patients with elevated urine iodine levels (over 200 ug/L) either from I.V. contrast or from dietary intake should have therapy postponed until levels return to normal (typically a 4 to 8 week delayed is recommended) [57,117]. The ATA guidelines indicate that urinary iodine levels usually return to normal in 4-8 weeks following IV contrast administration [119]. However, the precise period necessary to ensure that the previous contrast will have no effect on subsequent RAI therapy has not been precisely defined [119]. There remains some concern that even though urinary iodine levels have returned to normal, residual iodine in thyroid tissues could decrease the effectiveness of RAI therapy [119].
Other authors suggest that reduced iodine uptake following IV contrast may be independent of the amount of free iodide and related to contrast induced decreased Na/I symporter (the protein responsible for iodide uptake) expression on thyrocytes [117].
Diagnostic scanning following thyroidectomy:
In patients being considered for ablation therapy, a
pre-treatment diagnostic I-131 scan can be performed 5 to 6 weeks
following surgery to assess for the presence of metastatic
lesions. Either thyroid hormone withdrawl or thyrogen stimulation
can be used for patient preparation for the initial diagnostic
scan, as long as there is no evidence of metastatic disease [97].
For diagnostic scanning, some authors favor 20 minute spot images
of the head, neck, chest, abdomen, and pelvis. Other authors
perform total body scans with a moving camera. Whole body scans
with a table speed of 5 cm/ min. are probably comparable to spot
images, but this varies with detector size, given the same imaging
distance from the detector. Phantom studies should be performed to
determine the optimal table speed for individual systems. A
diagnostic study is not always required - particularly if the
total thyroidectomy has been performed by an experienced surgeon
in a low-risk patients who have no clinical evidence of tumor
after surgery [57,59].
Quantification of residual thyroid remnant tissue can be
performed at 24 hours following diagnostic tracer administration
using an uptake probe [126]. This procedure provides information
about the completeness of thyroid resection [126]. An RAIU of
1%-2% is consistent with an excellent resection and an RAIU of
< 5% is considered adequate [126]. RAIU in post thyroidectomy
patients also correlates with the likelihood of symptomatic
radiation thyroiditis [126]. The risk for thyroiditis is only 12%
for 37 MBq (1 mCi) delivered to the thyroid bed and the risk for
symptoms increases by 64% for each additional 37 MBq deposited in
the thyroid bed [126]. Severe thyroiditis was observed only in
those receiving > 73 MBq to the thyroid bed [126].
Planar whole body I-131 imaging has reported sensitivities of
45-75% and specificities of 90-100% for the detection of thyroid
cancer recurrences or metastases [85]. However, the anatomic
position and nature of foci of radioactivity can be difficult to
ascertain on planar images [92]. SPECT imaging has better contrast
resolution, but suffers from lack of low count statistics and lack
of anatomic landmarks [85]. However, SPECT/CT imaging has been
shown to aid in exam interpretation by detecting a greater number
of lesions than planar imaging (such as lymph node metastases
adjacent to residual thyroid tissue or the salivary glands), by
increasing reader confidence in the identification of physiologic
foci of tracer uptake (improving specificity with decreased
false-positive findings), and in improved localization of sites of
metastatic disease [82,84,85,98,100]. Authors have suggested
additional value of SPECT/CT imaging in up to 57-74% of cases and
that SPECT/CT findings can lead to changes in patient management
(such as I131 dose selection and in guiding surgical management)
[82,83,85,98,121]. Additionally, findings on the SPECT/CT exam can
aid in determining the likelihood for successful radio-iodine
ablation [98]. In one study, 94% of nodal metastatic deposits
smaller than 0.9mL were eliminated after radioablation, whereas
nodal mets exceeding this size were less likely to resolve (and
these patients may have been more appropriately guided to surgery)
[98].
Some limitations of SPECT /CT are related to the low intrinsic
activity of 131I scans that can produce very low count
images and this can result in image misregistration [82]. SPECT/CT
can also be used following high dose ablation therapy to aid in
lesion localization and risk of recurrence assessment- it is
particularly useful for differentiating thyroid bed activity from
adjacent lymph nodes [91].
False negative scans (up to 22% of patients) can occur in patients due to small lesion size, in non-iodine avid disease (20-30% of patients) such as Hurthle cell thyroid cancer, papillary thyroid cancer with unfavorable histology (tall cell, columnar, or cribiform variants), or poorly differentiated thyroid cancer (such as trabecular, insular, or solid variants) [98]. rH TSH stimulated whole body diagnostic exams have been shown to fail to detect remnant or cancer localized to the thyroid bed in 16% of patients in whom it was detected on a whole body scan following thyroid hormone withdrawal [132]. In addition, rh-TSH stimulated diagnostic scans failed to detect metastatic disease in 24% of patients in whom it was detected by a hormone withdrawal exam [132].
In patients with suspected metastases based on laboratory or
clinical findings that have previously undergone thyroid ablation
with I131, significantly more foci of metastatic disease may be
identified when patients are prepared with thyroid hormone
withdrawl, as opposed to thyrogen stimulation [97].
Thyroid Stunning: On imaging studies, thyroid stunning appears as an area of activity on the diagnostic scan which shows less activity on the patient's post-ablation scan [55,86]. Thyroid stunning can be seen in up to 19% of patients [55]. The etiology for stunning is not fully understood- some authors feel that the I-131 given for the diagnostic scan may exert a negative effect on the uptake or trapping of the therapeutic dose by residual thyroid bed tissue and functioning metastases due it's beta particle emission- following the initial diagnostic I-131 dose, "stunned" thyroid tissue loses its iodine trapping function partially or completely [38]. Other authors feel that stunning is not the result of the diagnostic I-131 dose, but rather the result of the therapeutic dose itself which results in results in a rapid loss of viable thyroid tissue and that the dying cells are incapable of retaining the accumulated iodide [65,86].
The importance of this stunning is that it may influence the rate of success of the radioablation treatment [54]. However, nearly everything about thyroid stunning is controversial- whether it actually exists, whether there is a dose threshold, and whether it actually effects therapeutic outcome [64]. To what extent stunning may limit the efficacy of 131I therapy has not been investigated in a prospective randomized study [69]. Stunning is believed to be a radiobiologic phenomenon and the degree of stunning depends on the absorbed radiation dose [38,42]. In fact, the higher the diagnostic dose used, the greater the possible subsequent decrease in uptake of the therapeutic dose. Most people feel that only 2 to 3 mCi of I-131 (and certainly no more than 5 mCi) should be used for the pre-ablation diagnostic scan. Some authors suggest only 1 mCi should be used for the diagnostic scan, because even a 3 mCi dose can exert a negative effect on ablation therapy [5]. Unfortunately, lower diagnostic doses can miss more metastatic lesions which would be detected with larger doses (up to 10 mCi). [6,7,8]. For patients that undergo diagnostic imaging following thyrogen stimulation, a diagnostic dose of 4 mCi has been suggested to compensate for the competitive inhibition exerted by the iodine content of T4 (levothyroxine) on the uptake of radioiodine [99,132]. Replacement of T4 with T3 (liothyronine) during the preparation period for rhTSH stimulation protocols has been proposed for decreasing the dilution effect of nonradioactive iodine on the uptake of radioiodine [132]. T3 has a lower iodine content per molecule (only 56.6% of T4 iodine content) and exerts an equivalent biologic effect with only 1/4 of the necessary T4 dose [132].
To decrease the effect of stunning, it may be beneficial to lengthen the period of time between the diagnostic scan and I-131 therapy to approximately one week [3]. However, other individuals feel that stunning is generally not observed until several days following the diagnostic scan and that the therapeutic dose should be given immediately following the diagnostic study [42].
One way that has been suggested to avoid the possibility of stunning is to use I-123 for diagnostic imaging prior to high dose I-131 treatment [64]. However, all radiation- including I-123, appears to exert a negative effect on the ability of thyroid cells to concentrate iodide and stunning can be seen following I-123 diagnostic imaging [86].
Although stunning is presumed to lessen the therapeutic effect of
I-131 ablation therapy and be associated with a lower success rate
for remnant ablation [28], it does not appear to have been
reported to be associated with a decreased patient survival. Other
authors have found no effect of stunning on the efficacy of 131I
for remnant ablation [55] and note similar final successful
ablation rates for both I-123 and low dose (74 MBq) I-131
preablation scanning [71]. Overall, stunning appears not to be a
problem at diagnostic doses of less than 2 mCi of I131 when the
I131 therapy is administered within 72 hours of the diagnostic
dose (stunning may be related to a true cytocidal effect of the
therapeutic dose) [102].
|
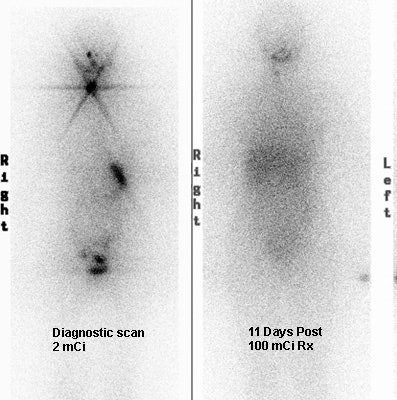
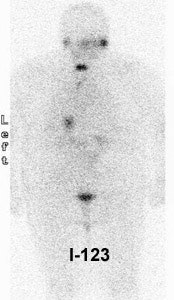
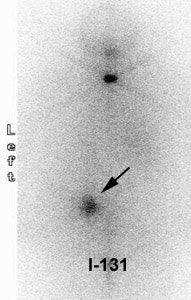
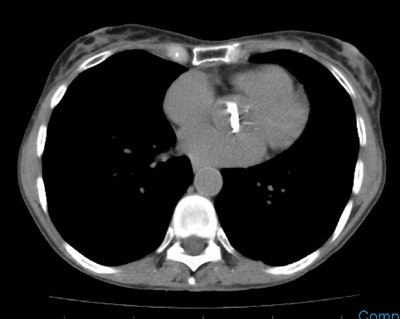
Thyroid Stunning: Diagnostic 2 mCi I-131 scan revealed neck bed activity (oral-pharyngeal, gut, and urinary bladder activity can also be seen). Following treatment with 100 mCi of I-131 the post-therapy scan demonstrated almost no evidence of tracer uptake in the neck indicative of thyroid stunning. Note hepatic activity consistent with breakdown of radiolabeled thyroxine. |
|
|
I-123 for diagnostic scanning:
Diagnostic quality pre-ablation scans can also be performed using I-123. A dose of 1.5 to 2 mCi is used and whole body and dedicated anterior and posterior neck/chest images are obtained at 24 hours [29]. By using I-123 the radiation dose to the thyroid gland is substantially decreased (by approximately 100 times) and I-123 has not been reported to cause thyroid stunning as it has no beta emission. Additionally, image quality is better with I-123 due to it's gamma energy of 159keV which is ideal for NaI crystal detectors and a greater photon flux (it gives approximately 20 times the count rate of I-131 for the same administered dose) [29,38].
I-123 scan findings are concordant with post-I-131 therapy scans in 93% of cases [29]. However, the earlier imaging with I-123 theoretically makes it less sensitive for detecting lesions with delayed uptake kinetics (ie- too short a time interval for optimizing tumor tracer uptake) [61,132]. Additionally, I-123 imaging may be less sensitive than I-131 scanning for the detection of metastases [40,126,132]. I-123 is also more expensive than I131 [29].
|
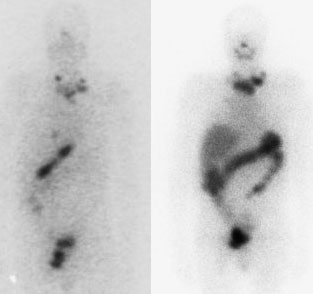
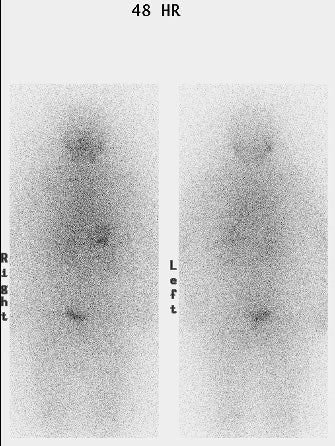
I-123 Diagnostic scan: The diagnostic scan on the left was performed using I-123. Extensive pathologic nodal uptake is seen within the lower neck and mediastinum. A separate focus of increased tracer accumulation is seen over the right upper abdomen (not seen on post-therapy scans- possibly due to superimposed liver activity). The post-I-131 therapy scan (right) demonstrates uptake in the nodal metastases and diffuse hepatic tracer activity due to metabolism of radiothyroxine. |
|
|
|
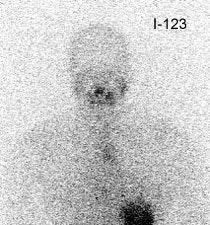
False negative I-123 diagnostic scan: The patient shown below had undergone thyroidectomy for papillary thyroid cancer and was presenting for evaluation prior to radio-iodine ablation therapy. The patient had a TSH level of greater than 90. The diagnostic scan on the left was performed using I-123. The exam revealed no evidence of neck bed activity and no metastases (the uptake in the chest was related to esophageal activity and cleared with water). Following treatment with 125 mCi of I-131 a 10 day post-therapy scan demonstrated a large amount of tracer activity in the thyroid bed and neck. Some authors are questioning whether I-123 scanning is comparable to an I-131 diagnostic exam [40]. |
|
|
|
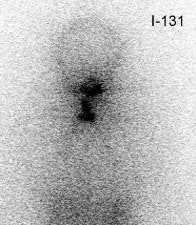
False negative I-123 diagnostic scan: The patient shown below had undergone thyroidectomy for papillary thyroid cancer and had a known metastasis to the left iliac bone. A diagnostic I-123 scan was performed to evaluate for extent of disease (posterior whole body image on left). The I-123 scan demonstrated neck bed uptake, but the iliac metastasis was not identified. Despite the negative diagnostic study, the patient received high dose I-131 therapy. The post therapy I-131 scan clearly revealed the iliac bone lesion (black arrow). |
|
|
I-131 Treatment Protocols for Thyroid Carcinoma:
I-131 therapy is scheduled to take place a minimum of 4 weeks following surgery [131]. For patients with metastatic disease, I-131 ablation therapy should ideally be performed within 180 days following surgery [95]. Delaying ablation for more than 180 days has been associated with overall decreased survival in patients with metastatic thyroid cancer [95].
Post operative Tg values of about 10 ng/mL after hormone withdrawal or 1 ng/mL after rhTSH stimulation achieve the best balance of sensitivity and specificity for predicting recurrent disease over time and poorer survival [132]. Unfortunately, very low Tg levels cannot be used to rule out the possibility of distant metastases as they can be identified in patients even with Tg levels below 1 ng/mL [132]. Iodine avid tissue can be identified in up to 20% of patients with an undetectable stimulated serum Tg level [132].
Patient preparation for optimal I-131 uptake includes 1-2 weeks of a low-iodine diet and adequate TSH stimulation (TSH > 30 mI/L) by either thyroid hormone withdrawal or recombinant human TSH stimulation (rhTSH 0.9 mg intramuscularly on two consecutive days) [131]. DIetary deprevation restricts iodine consumption to < 50 ug/d and is important for minimizing interference with I131 uptake [132]. Use of a low iodine diet has been shown to increase radioiodine uptake in the thyroid remnants by 65%, compared to controls [132]. Use of a low iodine diet has also been shown to be associated with increased likelihood for successful ablation (65% of LID patients compared to 48% of controls) [132]. Iodine deprivation is considered adequate when spot urine iodine is < 100 ug/L and optimal when urinary iodine is < 50 ug/L [132]. Patients on amiodarone should be placed on a different antoarrhythmic drug followed by serial urinary iodine measurements over 3-6 months after the drug is stopped to ascertain clearance of excess iodine [132].
For females of child bearing age a negative pregnancy test is required within 72 hours of I131 administration or before the first rhTSH injection, unless the patient has undergone a prior hysterectomy or is post-menopausal [131].
The activity of radioiodine used for ablation of thyroid remnants and treatment of metastatic disease is not standardized and several treatment options exist. In general, there is less need for radioactive iodine ablation in low-risk patients (small lesion under 1.5 cm) that have had a true total thyroidectomy and a greater need when large remnants are present or in patients who are at high risk for recurrence based on lesion size (over 1.5 cm), multicentricity, histology, age, or extrathyroidal extension [57]. Before receiving the therapeutic dose, the patient should be NPO for 2 to 4 hours, and should also remain NPO for 2-4 hours after dosing (to decrease the possibility of nausea and vomiting [34]. The administered dose must be within 10% of the ordered dose [34]. For patients with metastatic disease, I-131 ablation therapy should ideally be performed within 180 days following surgery [95]. Delaying ablation for more than 180 days has been associated with overall decreased survival in patients with metastatic thyroid cancer [95].
1- Aggressive/Restrained (Beierwaltes)
Fixed amounts of radioiodine are given based upon the presence and location of metastases [11]. This is a popular way for treatment as it is generally effective and simple to apply.
- Residual thyroid bed activity only: 100 mCi
- Regional Metastases (Cervical Nodes): 150-175 mCi
- Lung Metastases: 175-200 mCi (100-300 for pulmonary micrometastases [63])
- Skeletal Metastases: 200 mCi
Other authors suggest using 50-100 mCi (1.85-3.7 GBq) for adjuvant therapy, 100-150 mCi (3.7-5.6 GBq) for small volume locoregional disease, and 150-200 mCi (5.6-7.4 GBq) for treatment of advanced locoregional disease and/or small volume distant metastatic disease [132]. Identification of more wide-spread metastatic disease may lead to treatment dose escalation (>200 mCi) guided by dosimetry calculations [132].
2- Dosimetry:
Dosimetry is utilized to determine what activity the therapeutic dose should be based upon the individual patients radioiodine pharmacokinetics. The therapeutic dose is adjusted to compensate for patient to patient variability in the rate of iodine clearance [11]. Dosimetry is utilized in two instances- to maximize the dose of radioiodine given to the patient and in patients with altered iodine clearance.
High/Maximum dose: Dosimetry guided I-131 therapy allows the administration of the highest possible dose of I-131 in order to achieve maximum therapeutic benefit [48]. This treatment is based on the assumption that metastases may lose their ability to concentrate iodine over time due to repeated sub-therapeutic doses which permits surviving cells to regrow (resulting in de-differentiation with loss of iodine concentrating ability). Therefore, the largest and safest dose possible should be administered at the first therapy [48].
Dosimetry is used to determine the patients individual
radioiodine pharmakokinetics. The administered dose is then
individually tailored to keep the blood dose (bone marrow) just
below 200 rads/rem (2 Gy/Sv- although other centers use a blood
dose of 300 rads [48]) and limit whole body retention to less than
120 mCi (4.4 GBq) at 48 hours (or less than 80 mCi (3.0 GBq) if
there are diffuse lung metastases with uptake) [67,126]. In order
to achieve the highest rate of remission, pulmonary
micrometastases should be treated aggressively with repeat
radioiodine therapy every 6-12 months as long as the disease
continues to respond [63]. Patients with diffuse micrometastatic
disease to the lung may be difficult to treat due to the potential
for radiation-related pulmonary toxicity [48].
Using dosimetry, one study found lymph node metastases were
treated successfully in 74% of patients with a single
administration of I131 calculated to deliver at least 85 Gy [126].
For patients with nodal uptake only, and no uptake in other
metastases or in the thyroid bed, success was achieved in 86% of
patients with tumor doses of at least 140 Gy [126]. There was no
trend toward greater success with higher doses [126]. Lesions that
are calculated to receive less than 30-40 Gy may be considered for
alternative therapy [126].
Generally, patients receive about 300 mCi of radioiodine, but
doses up to 1 Ci have been administered [48]. The most common side
effect of high dose therapy is transient bone marrow depression
(thrombocytopenia and leukopenia) with a nadir seen between 3 to 5
weeks post-therapy [48]. Spontaneous recover can be expected over
the next 3 to 5 weeks following the nadir [48]. Mild-to-moderate
xerostomia is another common complication of very high dose
therapy [48]. It is important to note that there is no evidence to
suggest that this type of dosimetry-based therapy yields better
results compared to a fixed-activity regimen when the endpoint is
survival [67,116].
Altered iodine clearance: Conditions such as renal
failure, ascites, or pleural effusions can all result in prolonged
retention of I-131. Patients with diffuse lung metastases or bulky
functional metastases may also retain I-131 longer than usual as
they will produce large amounts of radiothyroxine [27]. Using
dosimetry the expected radiation dose to the whole body, blood,
and sites of functioning thyroid tissue (thyroid bed, mets) is
calculated. The therapeutic dose is then determined in order to
maximize its effectiveness and improve patient safety. Because a
dose of 1-2 mCi of I-131 is usually adequate for dosimetry, it can
be performed in conjunction with the pre-therapy diagnostic
examination.
Treatment in patients with renal failure: Treatment of patients
with renal failure is difficult due to the increased irradiation
to the patient arising from the long biologic residence secondary
to limited or absent urinary excretion (in patients with end stage
renal disease the effective half-life of I131 is more than 4 times
higher than in patients with normal renal function), the need for
a radiation protected hemodialysis unit (hemodialysis is not a
contraindication to I131 therapy), and management strategies for
the radioactive effluents produced during the first few dialysis
sessions after I131 therapy [99,128]. Studies have shown that the
mean fraction of I131 cleared after the first dialysis session
ranges from 50-72% [128].
For ablative or adjuvant treatment in hemodialysis patients, it
has been recommended to implement a 30% reduction in activity from
the nominal dose that would have been used [128]. For hemodialysis
patients with metastatic disease, dosimetry is recommended to
determine the amount of activity that should be administered
[128]. For the pre-treatment dosimetry exam, it is important that
dialysis sessions be performed under the exact same conditions as
to be done following therapy to avoid variations in patient
radiation exposure [128].
3- Low Dose [19]: ALARA
I-131 30 mCi (1110 MBq) is given repetitively as necessary in order to ablate the thyroid bed. Patients do not require hospitalization and up to 27% of patients will have successful ablation after only one dose [19]. Reduction in cost and patient inconvenience are factors which make this form of treatment attractive [3]. This type of treatment may best be considered for the very low risk patients: Age under 45 years, primary lesion less than 1.5 cm, no evidence of vascular, lymphatic, or capsular invasion, and a well-differentiated tumor.
4- Children:
In children, the I-131 dose should be adjusted to the child's age and disease stage [70]. For children under the age of 12 years a dose of 74.0-92.5 MBq/kg of body weight can be used; for older children a fixed dose of 2.2-3.7 GBq can be used if there are no distant metastases [70]. Other authors recommend 30-50 mCi for low risk disease, 150-175 mCi for higher-risk locoregional disease, and 175-200 mCi for known or suspected metastatic disease adjusted for weight based on a 70kg adult [131].
Guidelines for maximum dose administration
The guidelines regarding the maximum activity which can safely be administered are: [11]
1- Blood dose/bone marrow dose should be no more than 200 rads/rem (2 Gy/Sv)
This limit is set to reduce marrow toxicity. Frequently (90%) doses of this level are associated with mild, transient decreases in blood cell counts, but no instances of permanent suppression have been reported. In elderly patients, administered doses of more than 140 mCi or less rarely expose the blood to a dose of more than 2 Gy [66]. However, doses of 200-250 mCi frequently exceed this level and dosimetry should be considered in these cases - particularly if patients have iodine avid metastatic disease [66]. The bone marrow absorbed dose is lower after rhTSH-aided therapy compared to treatment following thyroid hormone withdrawal [56]. This is because clearance of the radioactive iodine is about 30% faster following rhTSH-aided therapy [66].
2- Retained whole body activity of no more than 120 mCi (4.440 GBq) at 48 hours (or 80 mCi (2.960 GBq) in patients with lung metastases to avoid potential pneumonitis and pulmonary fibrosis)
Guidelines for Outpatient Thyroid Remnant Ablation:
In the past, patients were required to be hospitalized and in
isolation after I-131 radioablation therapy until the dose rate
from the patient was <5 mrems/hr, or the retained activity in
the patient was <30 mCi. Patients can now be treated on an
outpatient basis providing that certain exposure limits are
maintained for individuals that may have contact with the patient
(limit of 0.5 rem (5 mSv) TEDE to an individual due to radiations
from the released patient). Click
here for a further discussion.
Measures which increase the radiation dose delivered to a metastatic lesion:
1- TSH manipulation
TSH should be greater than 30 uU/ml prior to I-131 ablation therapy [63,99]. Elevated TSH levels will stimulate iodine uptake in functioning mets. TSH stimulation with human TSH should be considered for patients with TSH values less than 30. If the TSH level is less than 30, functioning thyroid mets may not be identified on diagnostic scans, and may not accumulate sufficient I-131 during therapy. In order to ensure adequate elevation in TSH levels, T4 should be discontinued for at least 4 to 6 weeks prior to the scan/therapy (the half-life of T4 in the blood is about 1 week). T3 (Cytomel) should be discontinued for at least 10 to 14 days (the half-life of T3 in the blood is about 18-24 hours). The patients hypothyroid state (elevated TSH) results in decreased renal clearance of I-131 which increases the bioavailability for thyroid lesions [57]. This is not the case for euthyroid patients receiving rhTSH stimulation [57]. Unfortunately, the increased whole body retention associated with hypothyroidism also increases the radiation dose delivered to nonthyroid tissues- including the blood and bone marrow [57]. Decreased colonic motility associated with hypothyroidism can also increase the dose delivered to the colon- this can be minimized with the use of laxatives [57].
2- Low iodine diet
It is generally recommended that patients be placed on a low iodine diet for 7-14 days before treatment [,6399]. Daily dietary iodine intake is ideally maintained below 50 ug/day [63]. Patients should avoid seafood, salt, iodine containing medications, iodinated contrast medium, and dairy products. These measures will decrease the extracellular iodine pool and increase uptake of radioiodine by about 2.5 times. For patients that have received recent iodinated contrast (within 2-3 months) a urinary iodine level can be checked and should be below 50 microgm/24hours [99]
3- Lithium carbonate
Lithium suppresses the release of thyroid hormone from thyroid
tissue [11] without impairing iodine uptake [63] and it has been
found to prolong the biologic T1/2 of I-131 especially in tumors
with biologic half-lives of less than 6 days, with little effect
on whole body exposure [11]. When given for 1 week prior to
therapy, it may serve to increase the radiation dose delivered
to functioning thyroid tissue [34].
4- Tumor genetics
Mutations in BRAF (BRAF V600E), RAS, or RET (receptor tyrosine kinase) can stimulate mitogen-activated protein kinase (MAPK) signaling which inhibits expression of thyroid hormone biosynthesis genes, including the sodium-iodide symporter and thyroid peroxidase, which facilitate iodine uptake and organification, respectively, resulting in radioiodine refractory disease (RIRD) and rendering I-131 treatment less effective [112,122]. The BRAF V600E mutation is particularly associated with reduced expression of all thyroid-specific genes involved in iodine metabolism, resulting in variable decreased responsiveness to I131 therapy [132].
Studies have demonstrated that MAPK inhibition using MEK inhibitors (selumetinib, trametinib) or BRAF inhibitors (dabrafenib, verurafenib), or a combination of the two, can lead to restoration of sodium iodide symporter expression [122,132]. Tyrosine kinase inhibitors have been explored to restore iodine sensitivity in cases of radioiodine refractory disease [122]. TKI treatment is purely palliative and results in tumor shrinkage or prolonged progression free survival in a variable percentage of treated patients [122].
Selumetinib is a selective MEK inhibitor targeting MAPK used to increase sodium-iodide symporter expression and the agent has been shown to increase radioiodine uptake in previously refractory patients [112,122,132,133]. Following a 4 week course of therapy in RIRD patients, 60% demonstrated new or increased iodine incorporation and it was predicted that in 40% of patients the lesion absorbed radiation dose would be at least 20 Gy [122]. In another study, the treatment prolonged progression free survival from 5 to 11 months [122]. However, other authors indicate that there is significant inter- and intra-patient variability with respect to the effect of selumetnib on RAI uptake [133]. Secondary toxicities also required dose modification in 78% of patients [122].
Dabrafenib is a competitive inhibitor of BRAF kinase that has also been shown to improve response to radioiodine therapy in refractory patients with mtastatic disease [113]. In a study of BRAF V600E RIRD patients, 60% developed new radioiodine uptake following a 25 day course of treatment [122].
Lenvatinib is a multiple kinase inhibitor against the VEGFR1, 2, and 3 kinases. In a trial of patients with advanced DTC, the agent resulted in a response rate of 65%, compared to 2% in the placebo group [122]. However, because of toxicities, dosing had to be modified in 82% of patients [122].
rhTSH and I-131 ablation therapy:
rhTSH I-131 treatment is presently approved in the US for
ablation of thyroid remnants in patients with well-differentiated
thyroid cancer following near-total or total thyroidectomy without
evidence of metastatic disease [76]. The suggested dose is 0.9 mg
injected intramuscularly in the buttock for 2 consectutive days,
with administration of the treatment dose 24 hours following the
second injection [99]. Preliminary reports have indicated that
rhTSH preparation can be performed prior to ablation therapy for
thyroid remnants following surgery with comparable results to
patients treated following thyroid hormone withdrawal for residual
thyroid bed activity and recurrence [46,57,76]. This may be a
preferable method to prepare patients who may be unable to
tolerate thyroid hormone withdrawal [47].
Compared to hypothyroid patients, whole body retention of I-131 is decreased in euthyroid patients receiving rhTSH (in part due to slower renal clearance of I-131 in hypothyroid individuals) [50,51,79]. The mean effective half-life for I131 has been shown to be about 30% shorter for patients receiving rhTSH stimulation (10.5 hours versus 15.2 hours in similar low risk patient populations) [79]. This results in decreased patient irradiation, decreased blood dose and possibly less overall radiotoxicity [81], but may also result in decreased I-131 bioavailability [57,62]. Also- in euthyroid patients, the thyroid remnant is not iodine depleted as it is after a period of thyroid hormone withdrawal (which results in continuous TSH stimulation of secretion of organically bound iodine) [62]. Hence, remnant uptake has been shown to be less in patients receiving rhTSH compared to thyroid hormone withdrawal, although this was not statistically significant [62]. Therefore, issues remain with appropriate dose selection in patients receiving post rhTSH ablation treatment [46] and a larger therapeutic dose may be required [99]. One caveat is that remnant retention of iodine seems to be longer in rhTSH patients [62]. This may be related to persistently high TSH levels in hormone withdrawal patients which stimulates the release of organified radioiodine from the thyroid [62].
If patients elect to undergo ablation therapy following rhTSH stimulation additional preparation to stimulate I-131 uptake should also be employed [59]. This includes a 2 week low iodine diet and some authors recommend discontinuing thyroid hormone for 4 days prior and 1 day following the treatment [59,99]. Some authors also administer lithium for one week in order to enhance I-131 retention [59]. It may be reasonable to consider the administration of a higher dose of I-131 due to the more rapid tracer clearance and lower remnant uptake, but further studies will be required [62].
In patients with known persistent neoplastic foci, radioiodine treatment is preferably performed after thyroid hormone withdrawal [79]. Compared to normal thyroid tissue, metastatic thyroid cancer has low density and poorer functionality of NIS, therefore, TSH elevation over time is important to promote increased I131 uptake and retention in tumors [132]. The ATA guidelines state that rhTSH is an acceptable alternative to hormone withdrawal before remnant ablation or adjuvant therapy in low and intermediate risk patients without evidence of extensive lymph node involvement [127]. rhTSH is also registered for use in initial post operative I131 therapy in patients with up to N1M0 disease [132]. If extensive lymph node involvement is present without distant metastatic disease, rhTSH may be considered as an alternative to hormone withdrawal, but that more data is needed before making any recommendation for the use of rhTSH in high risk patients [127]. However, the ATA does recommend using rhTSH regardless of risk level for patients with comorbidities that preclude hormone withdrawal [127]. However, a European study with a mix of low, intermediate, and high risk patients found no difference in outcomes between rhTSH and hormone withdrawal during a 10 year follow-up period following RAIT [127]. Ultimately, the decision may be left to the patient after the risks and benefits of both methods are explained [127].
Follow-up Post Therapy Imaging
Follow-up whole body imaging is performed 7 to 10 days after high dose treatment with I-131- scans performed before this time may miss metastatic lesions. Post therapy scans provide incremental clinically relevant information by detecting new lesions not identified on diagnostic scans in up to 10-50% of patients [59,63,74]. The findings on the post-therapy scan can affect patient management in 9-15% of cases [63,74]. Post-therapy I-131 SPECT imaging with CT fusion can improve localization of metastatic lesions and aid in identification of physiologic sites of tracer uptake [52].
Normal sites of I-131 uptake:
Other than the thyroid bed, I-131 activity is also seen in the
choroid plexus, nasal mucosa/nasopharynx (may be asymmetric),
salivary glands, thymus, stomach, bowel, urinary bladder, and
breasts. Ectopic thyroid tissue may also be identified- a
thyroglossal duct remnant can be identified by its midline
location at the level of the hyoid bone [101]. Diffuse liver
uptake may be seen on post-therapy scans and is diagnostic of
thyroxine production [35]. (Thyroid hormone [T3,T4] is metabolized
in the liver and activity can be seen there after 3 to 7 days). In
post-ablation patients, liver activity suggests that I-131 has
been incorporated into thyroid hormone by functioning metastases.
A large amount of residual thyroid activity in the neck, however,
may produce similar findings. Focal oral uptake has been described
to localize to dental fillings possibly related to iodide ion
affinity for certain metals such as silver, gold, palladium, or
mercury [101]. Asymmetric salivary gland uptake can be seen- due
to tracer pooling in an obstructed gland (possibly the result of
prior I-131 radiation induced salivary duct srticture) or a result
of diminished function in the contralateral gland [101]. Benign
causes of thoracic and mediastinal radioiodine uptake include
swallowed radio-saliva or esophageal secretions in the esophagus,
Zenkers diverticulum, hiatal hernia, and gastric pull through
surgery [101]. Physiologic thymic activity (particularly in
children) can be seen and produces a characteristic "sail" or
"horse-shoe" configuration [101]. Areas of pulmonary infection can
show tracer uptake [101]. Radioiodine can also concentrate in
pleural or pericardial effusions and ascites due to passive
diffusion (and thrid spacing) [101]. Radioiodine uptake has been
reported in cystic structures including pericardial, bronchogenic,
liver, renal, ovarian, and sebaceous cysts [110]. The mechanism is
thought to be due to radioiodine entry into the cystic structure
through passive diffusion and retention in the cyst [110]. Chronic
sinusitis, skin abrasions, and post procedural sites can also
demonstrate tracer uptake due to inflammation [110].
There is considerable uptake within lactating breasts. It is
suggested that breast feeding be discontinued for several days
prior to treatment in order to ensure mammary secretory activity
has ceased in order to decrease the radiation dose to the breasts.
Breast feeding should be discontinued following treatment [12].
Breast uptake has been described in up to 6% of non-lactating
females and may be asymmetric. Breast uptake may be confused with
lung metastasis [13].
In the abdomen, focal tracer concentration can bs seen in Meckels
diverticulum containing ectopic gastric mucosa or in an inguinal
hernia containing bowel [101]. Tracer accumulation has also been
described in ovarian cysts [101].
|
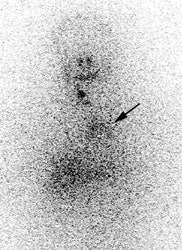
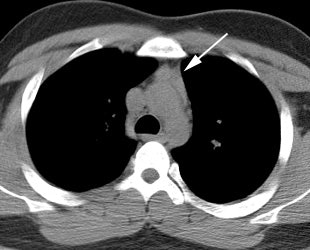
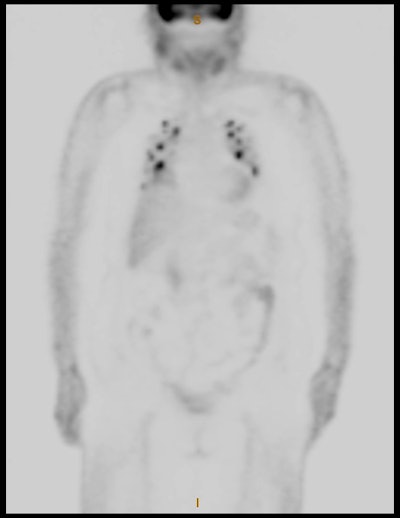
Thymic uptake on post therapy scan: The young male patient below received a 150 mCi ablation dose of I-131 for papillary thyroid cancer. On the post therapy scan, a triangular shaped area of tracer uptake was noted in the chest (black arrow). This was not seen on the pre-therapy diagnostic scan (not shown). CT imaging revealed normal thymus (white arrow). Radioiodine uptake in the thymus is thought to be related to tracer accumulation in Hassall bodies (Hassell corpusles) which are structurally similar to thyroid folliclesand sodium-iodide symporter expression [110]. Note the hepatic activity which is consistent with metabolism of radiolabeled thyroxine. Uptake in the neck is related to residual thyroid tissue. Normal naso/oropharyngeal activity is also seen. |
|
|
Radioiodine uptake has also been reported in other neoplasms
including lung cancer, Warthin tumor, gastric adenocarcinoma,
breast fibroadenoma, breast cancer, and neoplasms that contain
ectopic thyroid tissue such as struma ovarii [110].
Benefits of I-131 Therapy
1- Decreases local recurrence
Local recurrence rates are decreased by about 50% in patients treated with greater than 90mCi of I-131, compared to those treated with surgery alone. In patients with follicular cancer, RAI treatment has been shown to decrease the risk of local recurrence by 20 fold [43].
The only exception to this is in patients who are at lower risk for local recurrence to begin with: i.e.: age under 45y, no distant metastases, and primary tumor < 1.5cm [14]. Unfortunately, I-131 therapy has not been shown to statistically prolong survival in patients with only focal thyroid disease.
I-131 therapy produces a major decrease in recurrence risk- in patients with an excellent (complete) response, the risk of disease recurrence is 1%-4%, compared to an initial recurrence risk of 36-43% for intermediate risk patients and 68-70% for high-risk patients [131]. The clinical outcome in patients with a biochemically incomplete response is usually good: approximately 60% of patients have no evidence of disease on long term followup: 20% of patients will have persistently elevated thyroglobulin levels without a structural correlate, and 20% of patients will develop structurally identifiable disease over a 5-10 year followup period [131]. Patients with a biochemically indeterminate response generally do well: in 80-90% of patients, biochemical findings either remain stable or resolve over time with levothyroxine suppression therapy alone; however, up to 20% of these patients will eventually develop functional or structural evidence of disease progression and
I-131 treatment in combination with total thyroidectomy has also been shown to decrease the risk of local recurrence in adolescents and children [70].
2- Improves survival in patients following local recurrence
3- Prolongs survival in patients with lung or bone metastases
RAI treatment prolongs survival in patients with disseminated thyroid cancer, however, the rate of actual cure is low [15]. For patients with disseminated follicular thyroid cancer, the risk of dying from the disease is reduced to about one-sixth by the use of RAI therapy [43]. The efficacy of radioiodine therapy depends largely on the ability of distant metastases to take up radioiodine, and the 10 year survival rate can be as high as 56% in patients with an intense radioiodine accumulation, and as low as 10% in those who lose radioiodine avidity [111]. I-131 ablation has been reported to be less effective in patients younger than 40 years of age with metastases, however, these patients tend to live longer with metastatic disease.
In patients with lung metastases a micronodular (miliary) pattern of metastases is invariably related to good I-131 uptake and a better prognosis, while macronodular lesions (over 0.5 cm) frequently show poor uptake and have an associated worse prognosis [63]. Radioiodine therapy is of unlikely benefit if the lung nodules are identified only on FDG PET imaging [63].
Patients with bone metastases tend to have a higher mortality compared to patients with lung metastases [43]. Bone metastases typically have little or no response to I-131 therapy [14], however, I-131 treatment can be associated with some improved survival [63]. None-the-less, a surgical approach has become more popular, particularly if there is a solitary lesion and the patient is under the age of 45 years [45,63]. Patients with solitary bone metastases that undergo complete metastasectomy have a longer survival than those who have incomplete resection or non-operative treatment [45]. However, some authors have found that I-131 therapy can be used with curative intent in patients with bone metastases [31]. Patients with bone metastases that are most responsive to I-131 therapy tend to be younger (under 45 years of age) and have fewer bone lesions (less than 3 lesions) [31].
4- Eliminates the thyroid gland as source of thyroglobulin
I-131 ablation improves the sensitivity and specificity of
post-therapy follow-up whole body I-131 scans and serum
thyroglobulin assays. Thyroglobulin (Tg) is a glycoprotein that
is exclusively produced by the follicular cells of the thyroid
and metabolized in the liver [126]. Tg levels can become
significantly elevated after surgery due to manipulation of the
thyroid gland which results in enhanced release of Tg into the
circulation [126]. Tg decline following thyroidectomy with a
half-life of approximately 65 hours [126]. It takes
approximately 25 days after surgery for Tg levels to become a
reliable marker for residual thyroid tissue or metastatic
disease [126]. Thyroglobulin antibodies are a marker of thyroid
autoimmunity and are detected in about 20% of patients with DTC
[126]. The presence of TgAbs interferes with the reliable
measurement of Tg levels and can result in a falsely
low/undetectable Tg level [126].
Thyroglobulin appears to be the most sensitive test for determining the presence of persistent or recurrent well differentiated thyroid cancer. The thyroid gland is the only source of thyroglobulin production in the body. Thyroglobulin levels should be undetectable in post ablation patients. A thyroglobulin level drawn while the patient is on thyroid hormone replacement may be falsely depressed and should preferably be measured after the patient has been withdrawn from replacement (during maximal TSH stimulation). A problem that hampers accurate Tg measurement is interference in the Tg assay by Tg antibodies (TgAb) and heterophile antibodies (HAb) resulting in under- or overestimation of the serum Tg concentration [132].
On follow-up, a rising thyroglobulin level implies the presence
of functioning metastatic disease and will be elevated in 85-90%
of patients with mets. Patients with bone metastases tend to
have the highest Tg levels, followed by those with pulmonary
mets, and the least elevation is seen in patients with nodal
mets. Thyroglobulin also rises after ablation or treatment of
metastases with radioiodine and the levels tend to decrease more
slowly (over months). Note that Tg levels may not be produced or
secreted by thyroid cancers with aggressive or poorly
differentiated histology [126].
Acute Complications of I-131 Thyroid Ablation Therapy
1- Sialoadenitis/Xerostomia/Mucositis
The major and minor salivary glands concentrate iodine [26]. Sialadenitis, hyposalviation, and xerostomia are the most frequent adverse events from high-dose I-131 therapy [72,89,109]. Acute sialoadenitis is characterized by pain, tenderness, and swelling of the salivary glands. It occurs in 3-33% (up to 67% [75]) of patients and is dose related [1,78,87]. Chronic sialoadenitis can occur in 11-43% of treated patients [89]. An evaluation of the effects I131 on the salivary glands suggested that despite expressing sodium iodine symporters similar to those of thyroid tissue, salivary glands do not have thyroperoxidase and thus have an extremely short residence time for I131 [89]. That study suggested that I131 uptake in the salivary glands is highest at 1-2 hours after ingestion [89]. Afterward, I131 rapidly washes out from the glands and little I131 remains in the glands 24 hours after ingestion [89].
A decrease of more than 50% in saliva flow rate is generally regarded as the critical value for initiation of oral complaints [109]. Following therapy, between 17-37% of patients have subjective complaints of xerostomia during the first year, but many patients do not complain of clinical symptoms and up to 50% of patients will have objective evidence of reduced salivary function [26,87,108]. Objective reduced salivary gland function can persist beyond one year in 14% to 43% of patients [1,26], and objective evidence of xerostomia can be found in up to 14% of patients at 3 years post therapy [26]. In pediatric patients treated with radioiodine, salivary gland dysfunction can be found in up to 35.5% of patients [124]. Complete/persistent xerostomia occurs in only 2-4% [1,87]. The risk for persistent pain or xerostomia appears similar for patients treated with thyroid hormone withdrawn or thyrogen stimulation [87], however, in one study, patients prepared with rhTSH had a higher risk of salivary gland swelling (20% versus only 10% in those using thyroid hormone withdrawal) [87]. The risk of salivary dysfunction increases with increasing accumulated dose- although the relationship is not linear [1,26,124]. One study of pediatric patients, suggested salivary gland dysfunction is more likely to develop when patients have received cumulative doses of more than 200 mCi [124]. The observed alterations in salivary flow do not appear to be associated with the degree of semiquantitative salivary iodine uptake on diagnostic images [109]. Decreased salivary function may affect long term dental health in these patients as well [72].
To decrease salivary gland dose and reduce the incidence and
severity of sialoadenitis, increasing salivation by the use of
sour candies or gum chewing has been advocated [75] and the
usefulness may be increased by waiting 24 hours after I-131
administration [73]. The hard candy should not contain FD&C
red dyes 3 or 28 which are heavily iodinated [75]. Concurrent use
of anti-inflammatory agents such as motrin may aid in decreasing
the incidence of sialoadenitis [89]. Prophylactic use
of 2-(S)-(3-aminopropylamino) ethylphosphorothioic acid
(amifostine) has also been shown to protect the salivary glands
from the effects of radioiodine, however, availability of this
drug is currently limited [72,73]. At least one
article has suggested that vitamin E (800 IU/day for one week
before and 4 weeks after I-131) may have a protective effect on
salivary function, but the study consisted of only a small group
of patients and assessment of any potential for decreased I-131
efficacy for thyroid ablation was not evaluated [114]. Montelukast
is a leukotrine receptor antagonist that has been shown to have
salivary protective effects in mice [115].
Patients may also experience mild glossitis, oral mucositis, or
stomatitis (16% and 21%, respectively) with small oral ulcers and
dysgeusia [75,99]. Gentle brushing of thentire oral cavity with
oft toothbrush every 3-4 hours for 4-7 days while awake may help
to decrease oral mucositis [99].
2- Radiation parotiditis
3- Loss of or change in taste (Acute/Chronic)
Between 13% to 50% of patients will suffer from a transient loss of taste or smell. An unusual metal-like alteration in taste can occur [99]. A perminent alteration in taste occurs in only about 1% of patients [87].
4- GI symptoms
Nausea (up to approximately 30% of patients [122]) and vomiting (seen in <1%)
5- Minimal bone marrow suppression
Transient pancytopenia may occur following larger doses (150-200
mCi or if bone marrow dose exceeds 200 rem [2 Sv]) with a nadir at
about 6-10 weeks post therapy (up to 25% of patients, especially
the elderly [122]). Recovery is spontaneous.
Chromosomal abnormalities in peripheral lymphocytes are not uncommon (2-9%).
6- Radiation pneumonitis and pulmonary fibrosis
May rarely be a complication in patients with lung metastases. The risk can be decreased by restricting the whole body retention at 48 hours to less than 80 mCi.
7- Radiation Gastritis/Cystitis
8- Thyroid storm
May occur with extensive follicular mets due to release of large amounts of thyroid hormone- typically seen 2 to 10 days post treatment [34]
9- Transient amenorrhea/fertility
This occurs in older females and is not dose related. The typical dose to the ovaries is approximately 20 rads for a 100 mCi dose of I-131. Temporary ovarian failure has been described in about 25% of treated females, however, this may be related to other factors such as hypothyroidism and a perturbation of the pituitary-gonadal axis. I-131 therapy has not been shown to reduce fertility of treated patients or increase the risk of congenital abnormalities in their offspring [17,77,102]. Patient's offspring have also not been shown to be at increased risk for thyroid or non-thyroid cancers [77]. The rate of miscarriages is higher in women that have undergone surgical thyroidectomy for thyroid cancer- both before and following I131 treatment (increased from baseline of 10% to 20%) [77].
10- Decreased testicular function/fertility
Decreased testicular function is seen in 10 to 50% of male patients receiving doses greater than 100 mCi. Spermatogonia are the most sensitive testicular cells to external radiation. The cumulative dose to the testes after a standard 100 mCi dose is between 50 to 150 rad. Good hydration and frequent voiding (every 2 hours) can minimize the testicular dose. Transient (over 6 to 12 months) increased serum FSH levels are found in one-third of male patients treated with I-131. FSH is a sensitive marker of germinal cell failure. Decreased spermatic motility was also observed.
Permanent elevations in FSH are seen to develop in patients receiving multiple treatments with high cumulative doses. Testicular atrophy with absent spermatogenesis has been reported in 3 patients that received doses between 450 and 820 mCi in association with external radiation. Two of these patients had large functioning pelvic metastases.
The bottom line is that I-131 therapy for thyroid carcinoma is associated with transient impairment of testicular germinal cell function. The damage may become permanent in patients that receive high cumulative radiation doses [18]. Sperm storage before high dose I131 therapy may be considered [99].
11- Cerebral edema or spinal cord compression
Seen in patients with CNS mets
12- Radiation sickness
Dose > 200mCi. Headache, nausea, vomiting.
13- Transient alopecia
Most likely related to disturbances in thyroid hormone status, rather than a radiation effect [1].
14- Chronic or recurrent conjuctivitis, keratoconjunctivitis, decreased lacrimal function
About one-quarter of patients treated will have subjective complaints of xerophthalmia [1,26], and about 18% will have objective evidence of decreased lacrimal function after one year [26]. Objective evidence for persistent keratoconjuctivitis sicca can be found in up to 7.6% of patients three years following treatment [26]. Therefore, although decreased lacrimal function is common after therapy, it is generally a transient side effect [26].
Chronic Complications of I-131 Thyroid Ablation Therapy
*There is NO increased risk of thyroid tumors and no evidence of reduced fertility or genetic abnormalities in patients offspring due to I-131 therapy. Genetic defects have been detected in 1.8% of patients with high-dose treatment, a percentage equal to that in the normal population [31].
1- Secondary Tumors
It has been reported that there is a small risk of secondary
malignancies for patients that have received cummulative
activities of greater than 7.4 GBq of I131 [120]. Data from the
SEER database indicate most secondary malignancies were detected
within one year of RAI, suggesting a causual relationship to be
unlikely [120]. In general, patients with thyroid cancer have a
higher incidence of second primary tumors compared to the
general population [96]. Some authors feel that the risk of a
secondary tumor associated with I131 therapy is low and lacks
clinical impact [17,32]. The relative risk approximates 1.21 to
1.9 per 10,000 patient years compared to the general population
and is greatest for hematologic malignancies [31,96].
A- Leukemia
The risk for acute myelogenous leukemia is only minimally increased above the general population with a peak incidence 2 to 10 years (mean 42 months [48]) post therapy (0.5% increased risk). A recent article suggested the 10 year risk for AML or CML was 0.54% in patients that received surgery and RAI compared to 0.40% in surgery only patients [118]. Although one article suggested a 1 in 2000 risk for leukemia in patients receiving more than 100 mCi [120], affected patients at risk are generally above the age of 50 and have received a dose of approximately 900 mCi. The risk is greatest when this large dose has been given over a short period of time (6 to 12 weeks). These patients have usually received a blood dose greater than 200 rads. It may also be possible that patients with thyroid cancer are at an increased risk for this type of malignancy regardless of the type of therapy they receive. To minimize the risk for leukemia there should be a 1 year interval between therapies and a total cumulative dose of administered activity should not to exceed 800 mCi [11] It is important to note that the mortality from recurrence exceeds that from leukemia by 4 to 40 fold [43].
B- Bladder carcinoma
There may be a slight increased incidence (6x) of bladder carcinoma if total dose was given over a short interval and exceeds 1000 mCi. The latency period is between 15 to 20 years.
C- Breast carcinoma
There may be a slight increase in breast carcinoma (3x) if the
total dose was given over a short interval and exceeds 1000 mCi.
This may be related to a genetic disposition for both breast and
thyroid carcinoma or more careful clinical evaluation and
followup in these patients [104]. In a Taiwanese study, patients
with thyroid cancer were found to have a higher incidence of
breast cancer compared to controls, whether they received I131
therapy or not, although patients receiving treatment did have a
very slightly higher incidence (18.9 vs 17.7 per 10,000 person
years) [104].
D- Salivary carcinoma
There may be a very slightly increased risk for salivary tumors [17].
2- Hypoparathyroidism
Post I-131 treatment hypoparathyroidism is extremely rare.
Post-Therapy Hormonal Treatment
After I131 therapy, patients receive T4 therapy adjusted for risk stratification [132]. Thyroid hormone (T4) suppression therapy is effective in the management of differentiated thyroid carcinoma and doses sufficient to suppress TSH decrease the risk of recurrence. This is because well differentiate thyroid cancer responds to TSH stimulation, and grows more slowly in the absence of TSH.
For patients with structurally incomplete response, the serum TSH is suppressed to less than 0.1 mUI/L [131]. In the excellent treatment response category, the serum TSH is maintained at 0.5-2 mUI/L for intermediate risk patients and at 0.1-0.5 mUI/L for high risk patients [131]. For patients with biochemically indeterminate or incomplete responses (post therapy Tg remains detectable with evidence of structural disease), the recommended serum TSH target is 0.1-0.5 mUI/L [131,132].
Therapy response stratification [132]:
Excellent response- no clinical, biochemical, or structural evidence of disease; negative imaging and either suppressed Tg< 0.2nG/mL or stimulated Tg < 1 ng/mL
Biochemical incomplete response- abnormal Tg with suppressed Tg > 1 ng/mL or stimulated Tg > 10 ng/mL or rising anti-Tg antibody levels in the absence of localizable disease (negative imaging)
Structural incomplete response- persistent or newly identified locoregional or distant metastases (any Tg level)
Indeterminate response- non-specific biochemical (suppressed Tg 0.2-1 ng/mL or stimulated Tg 1-10 ng/mL or stable or
declining anti-Tg antibody levels) or structural findings that cannot be confidently classified as either benign or malignant.
Follow-up Post-Ablation Screening for Recurrent Thyroid Cancer:
Following treatment, surveillance for recurrence is a lifelong process. The risk of recurrence is related to the age at diagnosis, extent of primary disease, size of the primary lesion, and the presence of metastases [24]. The overall risk of recurrence of thyroid cancer is 20% [24] (15-30% [80]; up to 20% of patients with differentiated thyroid cancer develop locoregional recurrence [90]). However, in patients with an excellent response to therapy, the risk of disease recurrence is 1-4% [132]. This represents a major change in risk when a complete response is achieved (intermediate risk patients original risk for recurrence is estimated to be 36-43% and for high risk patients 68-70%) [132]. The clinical outcomes in patients with biochemical incomplete response are usually good- about 60% have no evidence of disease over long-term followup, 20% continue to have persistent abnormal Tg levels without structural correlates, and only 20% develop structurally identifiable disease over 5-10 year follow-up [132]. Patients with a biochemical indeterminate response generally do well- in 80-90% of patients the non-specific biochemical findings either remain stable or resolve over time with T4 suppression therapy alone; however, up to 20% of these patients will eventually develop functional or structural evidence of disease progression and require additional therapy [132]
Following surgery and thyroid ablation with I-131, thyroid cancer
patients are monitored for recurrence using serum thyroglobulin
(Tg) levels, neck ultrasound (commonly performed as the first
imaging test in patients with elevated Tg levels), and in some
cases with whole body I-131 scanning at increasing intervals.
Presently, the American Thyroid Association recommends that
following ablation, low risk patients with negative TSH-stimulated
thyroglobulin and negative cervical US do not require routine
follow-up whole body diagnostic iodine imaging [63].
However, up to 10% of recurrent and metastatic tumors may not be detected by Tg measurements alone [93]. In one study, 13% of previously ablated patients with recurrent disease had thyroglobulin levels < 1 ng/mL [125]. For recurrence in the thyroid bed, Tg levels have been shown to be less sensitive than US for the detection of recurrence [93]. Cervical US has also been found to be more sensitive than whole body iodine imaging for the detection of local recurrence (sensitivity 70-94% for US, versus 20-45% for WBS) [80].
The ATA recommends that following surgery, cervical US be performed to evaluate the thyroid bed and neck nodes at 6 and 12 months (and then annually for at least 3-5 years) [63]. Patients with initially higher stage tumors are at an increased risk for recurrence [93].
Findings concerning for recurrence in the thyroid bed include a greater than 6 mm hypoechoic lesion with internal vascularity [93]. Coarse or microcalcifications can also been seen within thyroid bed recurrences [93].
Features on US that are suggestive of metastatic lymph nodes include [80,88]:
1. Round shape (19-80% of malignant nodes versus 12-30% of reactive nodes)- sensitivity 55%, specificity 89%, accuracy 63%, PPV 94%, NPV 37%
2. Absence of an echogenic hilum (62-100% frequency of mets)- sensitivity 100%, specificity 48%, accuracy 88%, PPV 87%, NPV 100%
3. Hyperechogenicity within the node (58-86% frequency of mets)- sensitivity 59%, specificity 85%, accuracy 65%, PPV 93%, NPV 38%
4. Microcalcifications
5. Capsular/peripheral flow rather than hilar flow on color imaging
6. Cystic change (13-70% frequency of mets)- sensitivity 34%, specificity 96%, accuracy 48%, PPV 97%, NPV 30%. Papillary thyroid carcinoma most commonly shows cystic changes in lymph node metastases [88]. Pure cystic change is mostly found in young adults [88].
Microcalcifications and cystic changes are highly specific signs of metastases- with a specificity of up to 100% [80], as these findings are not observed in normal or reactive lymph nodes [88]. A short axis measurement of more than 7 mm for level II and more than 6 mm for the remainder of the cervical levels has a sensitivity of 93% and specificity of 83% for distinguishing metastatic from reactive nodes [80]. Location of the nodes are also important- almost half of reactive nodes are found in the upper 1/3 of the neck, and 2/3's of malignant nodes are found in the inferior neck (level IV) [80].
I-131 Follow-up Whole Body Imaging:
In patients that have undergone prior thyroidectomy and I-131
ablation, some authors have suggested that I-131 whole body
imaging should only be performed in patients with detectable
thyroglobulin levels (> 2 ng/mL) [94]. This is because the
negative predictive value of a negative stimulated thyroglobulin
level (less than 0.2 ng/mL) is very high [94]. The ATA states that
a diagnostic WBS mat be useful 6-12 months following RAIT in
patients with an intermediate or high risk of recurrence, if the
post therapy WBS demonstrated abnormal uptake outside of the
thyroid bed, if the post therapy scan demonstrated a large thyroid
remnant which limits assessment for nodal disease in the neck, and
in patients with anti-Tg antibodies at risk for false negative
serum Tg levels [127].
Neck ultrasound is also a very good initial examination in patients suspected of local recurrence.
Radioiodine imaging can be performed following levothyroxine withdrawl or recombinant human TSH (rhTSH stimulation) [44].
Standard levothyroxine withdrawn scanning:
Patients are withdrawn from thyroid hormone suppression therapy in order to elevate their TSH level (T4 should be discontinued for at least 4 to 6 weeks prior to the scan and T3 (Cytomel) should be discontinued for at least 10 to 14 days). However, adequate elevation in TSH (over 30) may be achieved in most patients simply by withdrawal of T4 for 3 weeks prior to the exam [53]. Unfortunately, this produces hypothyroidism which is associated with a negative impact on the patients life (about 50% of patients are unable to work in the week before and the week following scanning [44]) and thyroid hormone suppression withdrawal for a long period of time could potentially increase the risk for growth of metastatic foci. The overall sensitivity of low dose I-131 scanning for the detection of metastatic thyroid cancer range from 40-80%, and a specificity of 90-100% [44]. A low-iodine diet is generally recommended for 7 to 10 days before I-131 administration to enhance the sensitivity of disease detection [44].
Recombinant human thyroid stimulating hormone use in post-ablation imaging and therapy:
rhTSH and whole body scanning:
Recombinant human thyroid stimulating hormone treatment (rhTSH [Thyrogen]) has also been used prior to imaging post ablation patients for follow-up screening [36,37,41]. For this exam, patients do not need to discontinue thyroid hormone suppression therapy and therefore do not experience hypothyroid symptoms. The potential of tumor growth during long-term TSH stimulation is also avoided through the use of rhTSH [50]. Additionally, elevated thyroglobulin levels following rhTSH stimulation is a very sensitive marker for residual thyroid cancer [51]. A thyroglobulin level of greater than 2 ug/L following rhTSH administration can be associated with tumor recurrence in up to 75% of patients.
Patients receive an intramuscular injection of 0.9 mg (10 U) of rhTSH a day for two days [36]. Maximal serum TSH concentrations generally occur 24 hours after the second dose and remain elevated for approximately 4 days [37]. Side effects include nausea (6-16% of patients) and headache (9%) [36, 37,41]. An increase in serum thyroglobulin levels after rhTSH administration is a very sensitive indirect indicator of functioning thyroid tissue (ie: metastatic disease) [36].
Either I-131 or I-123 is given 24 hours after the second rhTSH dose and imaging can be performed at 48 hours after tracer administration (or at 24 hours for I-123) [36] (Dr. Stanley Goldsmith feels that additional scanning should be performed 24 hours after I-131 administration [verbal communication SNM meeting 2003] due to the decreased retention of iodine in patients undergoing Thyrogen stimulation [49]). Diagnostic I-131 scans performed after rhTSH administration may not identify metastatic disease due to: 1- higher iodine clearance in these euthyroid patients (there is a significant, at least 2-fold, increase in whole body retention of radioiodine at 48 hours after tracer administration in hypothyroid patients) and, 2- lack of sufficient duration of TSH stimulation to sites of metastatic thyroid tissue [36,37,49,50]. Superior I-131 scans have been observed in up to 29% of patients after conventional thyroid hormone withdrawal compared to exams performed using rhTSH stimulation [37]. Even when scans are performed using a larger dose of I-131 for the diagnostic study (4 mCi), up to 16% of of patients have superior imaging during thyroid hormone withdrawal [37]. For patients at moderate to high risk of harboring residual or recurrent thyroid cancer it would seem prudent to perform their diagnostic scan following thyroid hormone withdrawal [41]. Patients receiving rhTSH do not appear to be at an increased risk for developing antibodies to rhTSH even following repeat injections [37].
Monitoring thyroglobulin:
Thyroglobulin levels provide a means for detecting the presence
of recurrent disease and for monitoring response to therapy.
Thyroglobulin (Tg) is a prohormone for thyroid hormone and it is
produced exclusively by normal thyroid tissue and well
differentiated thyroid cancers [33]. Tg levels are measured after
the patient has been withdrawn from thyroid replacement therapy or
following rhTSH stimulation (or the Tg level may be falsely
depressed) [34,80]. Withdrawal/stimulated thyroglobulin
measurements have been shown to be more sensitive for the
detection of cancer recurrence compared to unstimulated
measurements (sensitivity hormone withdrawal - 96%, rhTSH
stimulated 93%, and unstimulated 78%) [80].
The American Thyroid Association recommends that an initial 6
month post ablation followup stimulated thyroglobulin procedure be
performed following hormone withdrawal- if low levels of
thyroglobulin are obtained, subsequent followup measurements can
be performed with rhTSH [80]. In a thyroid cancer patient who has
had a total thyroidectomy and has been successfully ablated with
I-131 the Tg level should be undetectable [34]. A rise in the Tg
level is almost always an indicator of recurrent/metastatic
disease [34].
Baseline thyroglobulin level following completion of initial
therapy (surgery and I131 ablation) also provide prognostic
information [80]. A Tg level of more than 2.0 ng/mL (either
following hormone withdrawl or stimulation) is predictive of
persistent disease or future metastases, while a level of less
than 0.5 ng/mL (1.0 ng/mL [90]) indicates absence of residual
tumor in 98% of patients [80,90]. Other authors indicate an
increased risk for recurrence in post thyroidectomy and ablation
patients with non-stimulated Tg levels > 0.1-0.2 ng/mL or
stimulated levels of 1-2 ng/mL [126]. Suppressed or stimulated Tg
levels of > 5-10 ng/mL increase the odds of detecting
metastatic disease on imaging studies [126].
Thyroglobulin antibodies (TgAb) can be found in about 25% (13-45% [3]) of patients with well differentiated thyroid cancer and can interfere with Tg measurements made by immunometric assay (IMA) and radioimmunoassay (RIA) [59]. On IMA testing (which is used by most commercial labs) TgAb's can result in a falsely negative Tg measurement [59]. RIA assays are less prone to TgAb interference, but the direction and magnitude of interference is less predictable and Tg levels can be falsely elevated [59]. TgAb's may disappear over time- generally within 2-4 years following thyroidectomy and complete ablation of any remaining thyroid tissue [59,61]. Another antibody that can interfere with Tg measurement is the serum heterophile antibody (HAB) [59]. These antibodies can result in falsely elevated Tg levels on IMA assays [59].
Circulating antibodies to thyroglobulin can interfere with radioimmunoassays that utilize the second antibody method to measure thyroglobulin levels causing either falsely high or low results. Between 13 to 45% of patients with well differentiated thyroid cancer have anti-Tg antibodies [3] which can decrease the sensitivity of serum Tg levels as an indicator for recurrent disease [16,34]. Following thryoidectomy and successful radioiodine ablation of any remaining thyroid tissue, anti-Tg levels often decline over time and are frequently undetectable by the thrid post-operative year [61]. Increasing levels of TgAb suggest persistent disease [63,80].
Compared with whole body I-131 scanning, thyroglobulin levels are a more sensitive test for detecting the presence of recurrent/metastatic disease [60]. In the absence of anti-thyroglobulin antibodies (and after the withdrawal of thyroid hormone), serum thyroglobulin levels have a sensitivity of about 80-90% for the detection of recurrent disease [3]. Unfortunately, thyroglobulin levels provide no localization information and thyroglobulin levels may not be elevated in patients with only regional lymph node metastases [23]. In one study comparing I-131 scanning and thyroglobulin levels [20], thyroglobulin levels were elevated (above 10 ng/ml) in 88% of patients with metastatic lesions, while the I-131 scan demonstrated lesions in only 55% of the patients with proven metastases. There were no instances of a positive I-131 scan and a negative serum thyroglobulin level [20]. Other authors have demonstrated similar sensitivities for I-131 scanning [44]. Patients with negative Tg levels, but positive iodine scans are seen less commonly, although this situation has been reported to occur in up to 35% of patients with positive iodine scans [61]. Generally, in these patients iodine uptake is limited to the thyroid bed, but up to 8.5% of patients can have metastatic disease [61].
Tumor dedifferentiation plays a role in cases in which thyroglobulin levels are elevated, but the I-131 scan is negative. Obviously, the ability of thyroid cancer cells to produce thyroglobulin is maintained for a longer period of time in the process of dedifferentiation, than is the ability to take up iodine [23]. This can be explained by the fact that the mechanisms necessary for iodine storage (ie: iodination, iodization, and synthesis of thyroglobulin) are much more complex than is the synthesis of thryoglobulin alone [23]. Diffuse hepatic uptake on post-ablation I-131 scans should be viewed as indirect evidence for the presence of metastatic disease, even if other foci of abnormal activity cannot be identified [23]. Hepatic activity is due to the breakdown of radioactive thyroid hormone by the liver [23]. Other factors such as the presence of micrometastases may also be responsible for negative I-131 scans in patients with elevated thyroglobulin levels.
Thyroglobulin levels (in conjunction with the I-131 scan results) also provide prognostic information. Patients with elevated thyroglobulin levels and a positive scan have the lowest overall mortality (44%), while those with metastases and a negative thyroglobulin level and negative scan have a mortality of 71%. Patients with elevated thyroglobulin levels and a negative scan have a mortality of 58%.
|
Dedifferentiated thyroid cancer metastases: The patient below had biopsy proven metastatic thyroid cancer to the lungs. A whole body exam performed 48 hours following the administration of 2mCi of I131 was essentially negative. A followup FDG PET scan demonstrated positive uptake within the lung lesions. Dedifferentiated thyroid cancer will lose it's ability to retain iodine, but the lesions can be identified on PET imaging. See the PET section for additional discussion. |
|
|
Sonography in the followup of thyroid cancer:
High resolution US has been shown to have superior sensitivity compared to whole-body I131 imaging for the evaluation of locoregional (neck) recurrence (94% versus 57%) [90]. Following thyroidectomy, the local inflammatory response results in proliferation of fibrofatty connective tissue which fills in the residual space left by the surgery [90]. On US, this fibrofatty tissue appears as an inverted triangular or flattened uniform hyperechoic area indented laterally by the carotid space, anteriorly by the strap muscles, and medially by the laryngotracheal structures [90]. Any hypoechoic nodule in the thyroid bed is suspicious for recurrence [90]. Note however, with radioactive iodine ablation, remnant thyroid tissue undergoes progressive fibrosis and can appear as a heterogenenous hypoechoic mass, but without vascularity [90].
Lymph node metastases are suggested if the following features are identified: round shape, absence of an echogenic hilum, microcalcifications, hyperechogenicity, peripheral color flow in the node, and cystic change [90].
Empiric treatment of high serum thyroglobulin levels:
Some groups advocate a trial of I-131 therapy (100-200 mCi) in
post-ablation patients with elevated thyroglobulin levels (Tg > 10 ng/mL after
withdrawal or > 5 ng/mL after rhTSH, but negative I-131
scans/imaging studies [3,24,33,126,129]. The rationale for this
approach is that these patients are presumed to have small volume
disease that will be effectively treated [60]. Between 20-64% of
these patients can have a positive post-therapy WBS following
their initial negative diagnostic exam [127]. Therapy can result
in a decrease in the thyroglobulin level in 50-72% of patients
(indicating that the treatment did have an effect on silent sites
of metastatic disease) and post-therapy scans may demonstrate
sites of metastases not evident on the pre-therapy scan in up to
59-62% of patients [21,33,60,126]. However, this blind type of
therapy can be considered to be futile in 38-50% of patients with
a tumor negative post therapy whole body scan [105]. Additionally,
there is no evidence of an overall survival improvement [127].
The exact Tg level that should prompt empiric I-131 therapy has
not been established, but the higher the Tg level, the more likely
the post-therapy I-131 scan will demonstrate foci of tracer uptake
[59]. A rising Tg level (doubling over a 12 month period) is a
more reliable indicator of recurrent tumor than a single Tg
measurement [59]. Therapy may be considered when the Tg level is
rising and reach 5 ug/L after rhTSH stimulation or 10 ug/L after
thyroid hormone withdrawl [59]. Empiric treatment has not yet been
demonstrated to improve overall patient survival [59]. In one
study, more than half of the patients who had negative results on
diagnostic scanning, but positive results on post therapy scanning
progressed shortly after receiving radioiodine therapy [103].
Further evaluation of patients with elevated thyroglobulin levels and negative I-131 scans should be performed with FDG PET imaging and with targeted sonographic evaluation of the neck [58]. Any enlarged lymph nodes identified sonographically can be biopsied. Cystic lymph nodes can also be associated with thyroid cancer metastases. These nodes can be aspirated and the contents sent for thyroglobulin levels (usually in 0.5 or 1 cc of normal saline).
I-131 for locoregional recurrence:
Small locoregional lymph node recurrences (less than 1 cm in diameter) can be treated with I-131 alone [57]. Larger lymph node metastases (over 1 cm) are usually only partially responsive to I-131 treatment and surgery should usually be undertaken as the first line of treatment [57]. In patients with locoregional recurrence some centers will perform dosimetry to determine the maximum safe dose the patient can receive; however, most centers will administer an empiric dose of 100-150 mCi [57]. A local or regional recurrence that persists after 2-3 courses of I-131 therapy should be surgically removed [57]. I-131 can be given to aid in surgical localization of the lesion via the use of a gamma probe [57].
The most common sites for distant metastases include the lungs, spine, and appendicular skeleton [57]. Complete resolution of distant metastatic lesions can be seen in up to 46% of patients [57]. Lung metastases generally have a better response than do bone mets [57]. Factors that predict a good response include young age and small volume disease [57]. Metastatic lesions that show a high avidity for for 18F-FDG appear to be resistent to high-dose radioiodine therapy [57]. Resolution of distant metastases on follow-up whole body I-131 scans should also be viewed cautiously as less differentiated components may only be evident on 18F-FDG PET imaging [57].
False positive sites of I-131 uptake:
False-positive scans have been reported in association with [34,39,44]:
1- Contamination by physiologic secretions: Saliva or suptum on the patients skin or handkerchief and esophageal activity.
2- Inflammatory and post-traumatic conditions: Foci of infection, severe burns, and post-traumatic superficial scabs.
3- Gastrointestinal activity: Meckle's diverticulum or duplication cysts with gastric mucosa
4- Non-thyroid malignancies and pathologic transudates
5- Other causes of false positive exams include: Pericardial effusion and breast uptake associated with lactation
Other agents used for post ablation screening:
The detection of non-iodine avid disease in patients with thyroid cancer and elevated thyroglogulin levels poses a clinical and therapeutic dilemma. A neck ultasound examination can detect regional adenopathy and can be a useful first step in the evaluation of these patients. Several other imaging agents can also be used to assess for recurrent/metastatic disease in these patients.
Thallium-201 is one agent that has been used for imaging patients with thyroid cancer. One of the advantages of thallium imaging is that the patient does not need to discontinue hormonal replacement therapy. Sensitivities for the detection of thyroid metastases has ranged from 45 to 94%, with specificities between 84 and 97% [44]. Thallium is less effective than I-131 in identifying bone, liver, and diffuse pulmonary metastases. Thallium also has poor sensitivity for the identification of residual neck bed non-tumor thyroid tissue [44]. Thallium is probably best used to identify the presence of cervico-mediastinal lymph node mets [44]. The exam may also be useful in patients with elevated thyroglobulin levels, but negative I-131 scans [44]. Due to the high background activity in the abdomen and pelvis thallium imaging in these regions is seldom useful. It is probably best to limit thallium scans to the head, neck, and chest in order to obtain higher count density images of these regions. [22] SPECT imaging is helpful in the chest and neck and can have up to a 25% increase in sensitivity compared to planar images [44].
Tc-Sestamibi and Tc-Tetrofosmin have also been used for thyroid cancer detection
FDG PET imaging in thyroid cancer- see PET tumor imaging section
Newer therapies for iodine-refractory disease:
Sorafenib, an agent that inhibits multiple tyrosine and Raf
kinases, has been approved for the treatment of iodine-refractory
metastatic thyroid cancer [103]. The agent has been shown to
improve median progression free survival by 5 months compared to
placebo (10.8 vs 5.8 months) [103]. Unfortunately, targeted
therapies are largely cytostatic rather than cytotoxic, have
associated side effects, and tend to lose their efficacy over time
[103].
REFERENCES:
(1) J Nucl Med 1998; Alexander C, et al.
Intermediate and long-term side effects of high-dose radioiodine
therapy for thyroid carcinoma. 39: 1551-1554
(2) J Nucl Med 1994; Samuel AM, et al. Radioiodine therapy for well-differentiated thyroid cancer: a quantitative dosimetric evaluation for remnant thyroid ablation after surgery. 35: 1944-50
(3) Semin Nucl Med 1995; Dworkin HJ, et al. Advances in the management of patients with thyroid disease. 25: 205-220
(4) Endorinology and Metabolism Clinics of North America 1990; Hay ID. Papillary thyroid carcinoma. 19: 545-571
(5) J Nucl Med 1998; Muratet JP, et al. Influence of scanning doses of Iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. 39: 1546-1550
(6) Endorinology and Metabolism Clinics of North America 1990; 19: 685-719
(7) Clin Nucl Med 1992; Park HM. Stunned thyroid after high-dose I-131 imaging. 17: 501-2 (No abstract available)
(8) Nuc Med Bio. 1986; Jeevanram. 13; 277,1986
(11) J Nucl Med 1992; Bushnell DL, et al. Complications, sequela and dosimetry of iodine-131 therapy for thyroid carcinoma. 33: 2214-21 (No abstract available)
(12) J Nucl Med 1994; Robinson PS, et al. Iodine-131 in breast milk following therapy for thyroid carcinoma. 35: 1797-1801
(13) J Nucl Med 1996; Hammami MM, Bakheet S. Radioiodine breast uptake in nonbreastfeeding women: clinical and scintigraphic characteristics. 37: 26-31
(14) Radiol Clin North Am 1993; Scott AM, Larson SM. Tumor imaging and therapy. 31: 859-79
(15) J Nucl Med 1995; Dottorini ME, et al. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma 36: 21-27
(16) Semin Nucl Med 1995; Dworkin HJ, et al. Advances in the management of patients with thyroid disease. 25: 205-220
(17) J Nucl Med 1995; Dottorini ME, et al. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma 36: 21-27
(18) J Nucl Med 1994; Pacini F, et al. Testicular function in patients with differentiated thyroid carcinoma treated with radioiodine.35: 1418-1422
(19) J Nucl Med 1993; Comtois R, et al. Assessment of the efficacy of iodine-131 for thyroid ablation. 34: 1927-1930
(20) J Nucl Med 1994; Lubin E, et al. Serum thyroglobulin and iodine-131 whole-body scan in the diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. 35: 257-262
(21) Thyroid 1994; Clark OH, et al. Managament of patients with differentiated thyroid cancer who have positive serum thyroglobulin levels and negative radioiodine scans. 4 (4): 501-505
(22) J Nucl Med 1988; Brendel AJ, et al. Thallium-201 imaging in the follow-up of differentiated thyroid carcinoma. 29: 1515-20
(23) J Nucl Med 2001; Schluter B, et al. Impact of FDG PET on patients with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131-I scan. 42: 71-76
(24) J Nucl Med 2001; Macapinlac HA. Clinical usefulness of FDG PET in differentiated thyroid cancer. 42: 77-78 (No abstract available)
(25) J Nucl Med 2001; Coover LR, et al. Therapeutic 131-I in outpatients: A simplified method conforming to the code of federal regulations, Tital 10, Part 35.75. 41: 1868-1875
(26) J Nucl Med 2001; Solans R, et al. Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. 42: 738-743
(27) J Nucl Med 2001; Sisson JC, Carey JE. Thyroid carcinoma with high levels of function: Treatment with 131I. 42: 975-983
(28) J Nucl Med 1998; Muratet JP, et al. Influence of scanning doses of iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. 39: 1546-1550
(29) J Nucl Med 2002; Shanker LK, et al. Comparison of 123I scintigraphy at 5 and 24 hours in patients with differentiated thyroid cancer. 43: 72-76
(30) J Nucl Med 2001; Hermanska J, et al. Improved prediction of therapeutic absorbed doses of radioiodine in the treatment of thyroid carcinoma. 42: 1084-1090
(31) Eur J Nucl Med 2001; Petrich T, et al. Outcome after radioiodine therapy in 107 patients with differentiated thyroid carcinoma and initial bone metastases: side-effects and influence of age. 28: 203-208
(32) Acta Oncologica 1992; Hall P, et al. Tumors after radiotherapy for thyroid cancer. 31: 403-407
(33) Eur J Nucl Med 2001; de Keizer B, et al. Efficacy of high therapeutic doses of iodine-131 in patients with differentiated thyroid cancer and detectable serum thyroglobulin. 28: 198-202
(34) J Nucl Med Technol 1993; Bender JM, Dworkin HJ. Iodine-131 as an oncology agent. 21: 140-150 (No abstract available)
(35) Thyroid and whole-body imaging. Charkes ND. In The Thyroid, 5th ed. Ed Ingbar and Braverman. Lippincott, Philadelphia, 1986. 458-478
(36) J Nucl Med 2001; David A, et al. Serum thyroglobulin concentrations and 131I whole-body scan results in patients with differentiated thyroid carcinoma after administration of recombinant human thyroid-stimulating hormone. 42: 1470-1475
(37) J Clin Endocrinol Metab 1999; Haugen BR, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawl for the detection of thyroid remnant or cancer. 84: 3877-3885
(38) J Nucl Med 2002; Park HM. 123I: almost a designer radioiodine for thyroid scanning. 43: 77-78 (No abstract available)
(39) J Nucl Med 2002; Regalbuto C, et al. false-positive findings on 131I whole body scans because of posttraumatic superficial scabs. 43: 207-209
(40) J Nucl Med 2002; Sarkar SD, et al. Comparison of 123I and 131I for whole-body imaging in thyroid cancer. 43: 632-634
(41) Radiographics 2002; Saremi F, et al. Pharmacologic interventions in nuclear radiology: indications, imaging protocols, and clinical results. 22: 477-490
(42) J Nucl Med 2002; Postgard P, et al. Stunning of iodide transport by 131I irradiation in cultured thyroid epithelial cells. 43: 828-834
(43) Cancer 2002; Chow SM, et al. Follicular thyroid carcinoma. Prognostic factors and the role of radioiodine. 95: 488-98
(44) Endocrinol Metab Clin North Am 2001; Haugen BR, Lin EC. Isotope imaging for metastaic thyroid cancer. 30: 469-492
(45) Surgery 2002; Stojadinovic A, et al. The role of operations for distantly metastatic well-differentiated thyroid carcinoma. 131: 636-643
(46) J Nucl Med 2002; Robbins RJ, et al. A Retrospective Review of the Effectiveness of Recombinant Human TSH as a Preparation for Radioiodine Thyroid Remnant Ablation. 43: 1482-1488
(47) J Endocrinol Invest 2002; Berg G, et al. Radioiodine ablation and therapy in differentiated thyroid cancer under stimulation with recombinant human thyroid-stimulating hormone. 25: 44-52
(48) J Nucl Med 2003; Dorn R, et al. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. 44: 451-456
(49) J Nucl Med 2003; Sisson JC, et al. Increasing efficacy and safety of treatments of patients with well-differentiated thyroid carcinoma by measuring body retentions of 131I. 44: 898-903
(50) J Nucl Med 2003; Menzel C, et al. rhTSH stimulation before radioiodine therapy in thyroid cancer reduces the effective half-life of 131I. 44: 1065-1068
(51) J Nucl Med 2003; Robbins RJ, Pentlow KS. Coming of age: recombinant human thryoid-stimulating hormone as a preparation for 131I therapy in thyroid cancer. 44: 1069-1071
(52) J Nucl Med 2003; Yamamoto Y, et al. Clinical usefulness of fusion of 131I SPECT and CT images in patients with differentiated thyroid carcinoma. 44: 1905-1910
(53) J Nucl Med 2004; Grigsby PW, et al. Preparation of patients with thyroid cancer for 131I scintigraphy or therapy by 1-3 weeks of thyroxine discontinuation. 45: 567-570
(54) J Nucl Med 2004; Lassmann M, et al. Impact of 131I diagnostic activities on the biokinetics of thyroid remnants. 45: 619-625
(55) J Nucl Med 2004; Dam HQ, et al. 131I therapeutic efficacy is not influenced by stunning after diagnostic whole-body scanning. 232: 527-533
(56) J Nucl Med 2004; de Keizer B, et al. Bone marrow dosimetry and safety of high 131I activities given after recombinant human thyroid-stimulating hormone to treat metastatic differentiated thyroid cancer. 45: 1549-1554
(57) J Nucl Med 2005; Robbins RJ, et al. The evolving role of 131I for the treatment of differentiated thyroid carcinoma. 46: 28S-37S
(58) Radiol Clin N Am 2004; Zhuang H, et al. Investigation of thyroid, head, and neck cancers with PET. 42: 1101-1111
(59) J Nucl Med 2005; Mazzaferri EI. Empirically treating high serum thyroglobulin levels. 46: 1079-1088
(60) J Nucl Med 2005; Ma C, et al. Is empiric 131I therapy justified for patients with positive thyroglobulin and negative 131I whole body scan results? 46: 1164-1170
(61) J Nucl Med 2005; Ma C, et al. Possible explanations for patients with discordant findings of serum thyroglobulin and 131I whole-body scanning. 185: 1473-1480
(62) J Nucl Med 2006; Hanscheid H, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. 47: 648-654
(63) Thyroid 2006; Cooper DS, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. 16: 1-25
(64) J Nucl Med 2006; Woolfenden JM. Thyroid stunning revisited. 47: 1403-1405
(65) J Nucl Med 2006; Sisson JC, et al. The so-called stunning of thyroid tissue. 47: 1406-1412
(66) J Nucl Med 2006; Tuttle RM, et al. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. 47: 1587-1591
(67) J Nucl Med 2006; Sgouros G, et al. Lung toxicity in radioiodine therapy of thyroid carcinoma: development of a dose-rate method and dosimetric implications of the 80-mCi rule. 47: 1977-1984
(68) J Nucl Med 2006; Song H, et al. Lung dosimetry for radioiodine treatment planning in the case of diffuse lung metastases. 47: 1985-1994
(69) J Nucl Med 2007; Lundh C, et al. Reduced iodide transport (stunning) and DNA synthesis in thyrocytes exposed to low absorbed doses from 131I in vitro. 48: 481-486
(70) J Nucl Med 2007; Handkiewicz-J D, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. 48: 879-888
(71) J Nucl Med 2007; Silberstein EB. Comparison of outcomes after 123I versus 131I preablation imaging before radioiodine ablation in differentiated thyroid carcinoma. 48: 1043-1046
(72) J Nucl Med 2007; Walter MA, et al. The dental safety profile of high-dose radioiodine therapy for thyroid cancer: long term results of a longitudinal cohort study. 48: 1620-1625
(73) J Nucl Med 2005; Nakada K, et al. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? 46: 261-266
(74) Radiology 2008; Donahue KP, et al. INitial staging of differentiated thyroid carcinoma: continued utility of posttherapy 131I whole-body scintigraphy. 246: 887-894
(75) J Nucl Med 2008; Silberstein EB. Reducing the incidence of 131I-induced sialadenitis: the role of pilocarpine. 49: 546-549
(76) J Nucl Med 2008; Tuttle RM, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawl. 49: 764-770
(77) J Nucl Med 2008; Garsi JP< et al. Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to ovaries and outcome of pregnancies. 49: 845-852
(78) J Nucl Med 2008; Hay ID, et al. Perspective: the case against radioiodine remnant ablation in patients with well-differentiated thyroid carcinoma. 49: 1395-1397
(79) J Nucl Med 2008; Remy H, et al. 131I effective half-life and dosimetry in thyroid cancer patients. 49: 1445-1450
(80) Radiology 2008; Johnson NA, Tublin ME. Postoperative surveillance of differentiated thyroid cancer: rationale, techniques, and controversies. 249: 429-444
(81) J Nucl Med 2008; Rosario PW, et al. Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicity. 49: 1776-1782
(82) AJR 2008; Wong KK, et al. Incremental value of diagnostic 131I SPECT/CT fusion imaging in the evaluation of differentiated thyroid carcinoma. 191: 1785-1794
(83) J Nucl Med 2008; Chen L, et al. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. 49: 1952-1957
(84) J Nucl Med 2009; Schmidt D, et al. Impact of 131I SPECT/spiral CT on nodal staging of differentiated thyroid carcinoma at the first radioablation. 50: 18-23
(85) J Nucl Med 2009; Spanu A, et al. 131I SPECT/CT in the follow-up of differentiated thyroid carcinoma: incremental value versus planar imaging. 50: 184-190
(86) J Nucl Med 2009; Lundh C, et al. Radiaiton-induced thyroid stunning: differential effects of 123I, 131I, 99mTc, and 211At in iodide transport and NIS mRNA expression in cultured thyroid cells. 50: 1161-1167
(87) J Nucl Med 2009; Grewal RK, et al. Salivary gland side effects commonly develop several weeks after initial radioactive iodine ablation. 50: 1605-1610
(88) AJR 2010; Sohn YM, et al. Diagnostic approach for evaluation of lymph node metastases form thyroid cancer using ultrasound and fine-needle aspiration biopsy. 194: 38-43
(89) J Nucl Med 2010; Liu B, et al. Influence of vitamin C on salivary absorbed dose of 131I in thyroid cancer patients: a prospective, randomized, single-blind, controlled trial. 51: 618-623
(90) AJR 2010; Ko MS, et al. Normal and abnormal sonographic findings at the thyroidectomy site in postoperative patients with thyroid malignancy. 194: 1596-1609
(91) J Nucl Med 2010; Grewal RK, et al. The effect of posttherapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. 51: 1361-1367
(92) AJR 2010; Wong KK, et al. Staging of differentiated thyroid carcinoma using diagnostic 131I SPECT/CT. 195: 730-736
(93) AJR 2011; Kamaya A, et al. Recurrence in the thyroidectomy bed: sonographic findings. 196: 66-70
(94) J Nucl Med 2011; de Meer SG, et al. The role of routine
diagnostic radioiodine whole-body scintigraphy in patients with
high-risk differentiated thyroid cancer. 52: 56-59
(95) J Nucl Med 2011; Higashi T, et al. Delayed initial
radioactive iodine therapy resulted in poor survival in patients
with metastatic differentiated thyroid carcinoma: a retrospective
statistical analysis of 198 cases. 52: 683-689
(96) J Clin Endocrinol Metab 2008; Brown AP, et al. The risk of
second primary malignancies up to three decades after the
treatment of differentiated thyroid cancer. 93: 504-515
(97) J Nucl Med 2012; Van Nostrand D, et al. Recombinant human
thyroid-stimulating hormone versus thyroid hormone withdrawl in
the identification of metastasis in diifferentiated thyroid cancer
with 131I planar whole-body imaging and 124I
PET. 53: 359-362
(98) J Nucl Med 2012; Avram AM. Radioiodine scintigraphy with
SPECT/CT: an important diagnostic tool for thyroid cancer staging
and risk stratification. 53: 754-764
(99) J Nucl Med 2012; Siberstein EB, et al. The SNNMI practice
guideline for therapy of thyroid disease with 131I
3.0*. 53: 1633-1651
(100) Radiology 2012; Maruoka Y, et al. Incremental diagnostic
value of SPECT/CT with 131I scintigraphy after
radioiodine therapy in patients with well-differentiated thyroid
carcinoma. 265: 902-909
(101) Radiographics 2013; Glazer DI, et al. SPECT/CT evaluation
of unusual physiologic radioiodine biodistributions: pearls and
pitfalls in image interpretation. 33: 397-418
(102) J Nucl Med 2014; Avram AM, Shulkin BL. Thyroid cancer in
children. 55: 705-707
(103) J Nucl Med 2014; Pryma DA, Mandel SJ. Radioiodine therapy
for thyroid cancer in the era of risk stratification and
alternative targeted therapies. 55: 1485-1491
(104) J Nucl Med 2016; Lin CY, et al. Risk of breast cancer in
patients with thyroid cancer receiving or not receiving 131I
treatment: a nationwide population-based cohort study. 57: 685-690
(105) J Nucl Med 2016; Kist JW, et al. 124I PET/CT to
predict the outcome of blind 131I treatment in
patients with biochemical recurrence of differentiated thyroid
cancer: results of a multicenter diagnostic cohort study
(THYROPET). 57: 701-707
(106) J Nucl Med 2016; Wierts R, et al. Dose-response
relationship in differentiated thyroid cancer patients unedrgoing
radioiodine treatment assessed by means of 124I
PET/CT. 57: 1017-1032
(107) J Nucl Med 2016; 124I PET assessment of
response of bone metastases to initial radioiodine treatment of
differentiated thyroid cancer. 57: 1499-1504
(108) AJR 2016; Roh SS, et al. Association of xerostomia and
ultrasonographic features of the major salivary glands after
radioactive iodine ablation for papillary thyroid carcinoma. 207:
1077-1081
(109) J Nucl Med 2016; Klein Hesselink EN, et al. Effects of
radioiodine treatment on salivary gland function in patients with
differentiated thyroid carcinoma: a prospective study. 57:
1685-1691
(110) Radiographics 2017; Chudgar AV, Shah JC. Pictorial review
of false-positive results on radioiodine scintigrams of patients
with differentiated thyroid cancer. 37: 298-315
(111) J Nucl Med 2017; Yang X, et al. TERT promoter mutation
predicts radioiodine-refractory character in distant metastatic
differentiated thyroid cancer. 58: 258-265
(112) N Engl J Med 2013; Ho AL, et al. Selumetinib-enhanced
radioiodine uptake in advanced thyroid cancer. 368: 623-632
(113) Thyroid 2015; Falchook GS, et al. BRAF inhibitor dabrafenib
in patients with metastatic BRAF-mutant thyroid cancer. 25: 71-77
(114) Nucl Med Commun 2013; Fallahi B, et al. Does vitamin E
protect salivary glands from I-131 radiation damage in patients
with thyroid cancer? 34: 777-786
(115) Nucl Med Commun 2013; Koca G, et al. The efficacy of
montelukast as a protective agent against 131I-induced
salivary gland damage in rats: scintigraphic and histopathologic
findings. 34: 507-517
(116) J Nucl Med 2017; Deandreis D, et al. Comparison of empiric
versus whole-body/-blood clearance dosimetry-based approach to
radioactive iodine treatment in patients with metastases from
differentiated thyroid cancer. 58: 717-722
(117) J Nucl Med 2018; Vassaux G, et al. Iodinated contrast
agents perturb iodide uptake by the thyroid independently of free
iodide. 59: 121-126
(118) J Clin Oncol 2017; Molenaar RJ, et al. Risk of hematologic
malignancies after radioiodine treatment of well-differentiated
thyroid cancer.
(119) J Nucl Med 2018; Tuttle RM. Controversial issues in thyroid
cancer management. 59: 1187-1194
(120) J Nucl Med 2018; Schmidt M, et al. A matter of controversy:
is radioiodine therapy favorable in differentiated thyroid
carcinoma? 59: 1195-1201
(121) J Nucl Med 2018; Spanu A, et al. Role of diagnostic 131I
SPECT/T in long term followup of patients with papillary thyroid
microcarcinoma. 59: 1510-1515
(122) J Nucl Med 2019; Kreissl MC, et al. Current treatment
strategies in metastasized differentiated thyroid cancer. 60: 9-15
(123) Radiology 2008; Johnson NA, Tublin ME. Postoperative
surveillance of differentiated thyroid cancer: rationale,
techniques, and controversies. 249: 429-444
(124) J Nucl Med 2019; Selvakumar T, et al. Long-term effects of
radioiodine treatment on salivary gland function in adult
survivors of pediatric differentiated thyroid carcinoma. 60:
172-177
(125) J Nucl Med 2019; Bacher R, et al. Thyroid uptake and
effective half-life of radioiodine in thyroid cancer patients at
radioiodine therapy, and follow-up whole-body scintigraphy either
in hypothyroidism or under rhTSH. 60: 631-637
(126) J Nucl Med 2020; Donohoe KJ, et al. Appropriate use
criteria for nuclear medicine in the evaluation and treatment of
differentiated thyroid cancer. 61: 375-396
(127) AJR 2020; Ciarallo A, Rivera J. Radioactive idoine therapy
in differentiated thyroid cancer: 2020 update. 215: 285-291
(128) J Nucl Med 2020; Vermandel M, et al. Management of patients
with renal failure undergoing dialysis during 131I
therapy for thyroid cancer. 61: 1161-1170
(129) J Nucl Med 2021; Cheng L, et al. Unexplained hyperthroglobulinemia in differentiated thyroid cancer patients as an indication for radioiodine adjuvant therapy: a prospective multicenter study. 62: 62-68
(130) J Nucl Med 2021; Seifert R, et al. Minimal extrathyroidal extension in papillary thyroid microcarcinoma is an independent risk factor for relapse through lymph node and distant metastases. 62: 1702-1709
(131) J Nucl Med 2022; Avram AM, et al. Management of differentiated thyroid cancer: the standard of care. 63: 189-195
(132) J Nucl Med 2022; Avram AM, et al. SNMMI procedure standard/EANM practice guideline for nuclear medicine evaluation and therapy of differentiated thyroid cancer: abbreviated version. 63: 15N-35N
(133) J Nucl Med 2023; Taprogge J, et al. The role of pretherapy quantitative imaging and dosimetry in radioiodine therapy for advanced thyroid cancer. 64: 1125-1130