Myocardial Viability Imaging
Background
Patients with chronic coronary artery disease and severe left ventricular dysfunction (left ventricular ejection fractions below 30% to 35%) have poor survival. Patients with ejections fractions greater than 50% have a 4-year survival rate of 92%, compared to only 57% for those with ejection fractions below 35% [34]. In patients with left ventricular ejection fractions (LVEF's) below 30% and predominant heart failure symptoms, coronary revascularization can improve LV function, heart failure symptoms, and long-term prognosis when compared with medical treatment alone [25,30]. A metaanalysis revealed an almost 80% reduction in annual mortality (from 16% to 3.2%) for patients with viable myocardium that underwent revascularization as compared to medical therapy (lack of viability was associated with no difference in outcome) [84]. However, the recent STICH trial did not confirm an impact of viability on patient outcome with revascularization compared to optimal medical therapy (although the presence of viability was associated with overall better survival) [85]. It has been estimated that between 25-40% of patients with chronic coronary artery disease and left ventricular (LV) dysfunction have the potential for significant improvement in left ventricular function following revascularization [30]. Unfortunately, patients with reduced LVEF's are at a greater risk for surgical mortality from coronary artery bypass graft (CABG) (about 4% mortality) and physicians are reluctant to recommend surgery if the prospects for benefit are limited.
Because post-operative improvement in LVEF has been related to the preoperative identification of viable myocardial tissue, viability is a crucial factor when considering chronic congestive heart failure (CHF) patients for coronary artery bypass grafting [30]. It has been estimated that in patients with chronic ischemic LV dysfunction, more than 50% of dysfunctional segments can be shown to be viable [73]. Patients with a greater percentage of viable tissue are more likely to have improved LVEF, decreased symptoms, and improved survival [25,49]. Patients with evidence of viability involving 18% or more of the LV myocardium have been shown to have the greatest improvement in functional status [59]. In a meta-analysis, patients with predominantly viable myocardium who were treated medically had a cardiac death rate of 16% per year [55]. Patients with predominantly viable myocardium who underwent revascularization had an 80% lower mortality rate and a cardiac event rate of only 3.2% per year [55]. In contrast, no difference in cardiac death rate was seen in patients with predominantly non-viable myocardium who underwent revascularization versus medical therapy (7.7% versus 6.2%, respectively) [55]. However, even patients that do not demonstrate an improvement in ejection fraction, can have a survival benefit following revascularization- possibly related to a reduced incidence of subsequent ischemic events [49]. Of course, the presence of viable myocardial tissue is just one factor to consider when evaluating patients for revascularization surgery. Although restoration of myocardial function is unlikely in the absence of viable tissue, revascularization can be associated with other benefits, such as relief of ischemia, pain relief, stabilization (or reversal) of remodeling, and decreased incidence of arrhythmias [59]. In patients with extensive scar tissue, bypass surgery could be combined with resection of non-viable scar tissue [73]. Several studies have demonstrated that surgical reduction of dyskinetic (aneurysm) and akinetic regions may reduce wall stress and improve geometry and LV function in some patients [73]. The bottomline is that there is "a body of literature demonstrating that patients with severe LV dysfunction after MI, severe angina (with or without heart failure), and bypassable coronary arteries show a survival benefit from revascularization compared to medical therapy alone" [49].
Immediately following revascularization there may be a period of stunning in which LV function deteriorates. Imaging 2 to 3 months after the procedure is probably more representative of true post-op LV function. Improvement in regional function may occur without improvement in overall ejection fraction. LV size is also an important determinant of the potential for recovery of function--little to no improvement is seen in patients with marked LV dilatation [5]. Terms which are used in the discussion of myocardial viability include stunned and hibernating myocardium.
Recently, intramyocardial injection of bone marrow cells (progenitor cells) has been proposed as a therapeutic option for patients with chronic ischemic heart disease [68]. The marrow cells are thought to promote angiogenesis which results in increased myocardial perfusion and improved function [68]. In the area of infarction to be treated, it is likely that some residual viability may be a prerequisite for new myocyte formation from the stem cells [78]. The residual surviving microcirculation and the myocytes may serve as an impulse and substrate for the myocardial regeneration induced by the stem cell transplantation [78]. Responders to the treatment have been shown to have a significantly higher MIBI uptake in the infarcted area compared to non-responders (baseline of less than 30% MIBI activity) [78]. PET studies have demonstrated a significant reduction in the amount of myocardium with a perfusion-metabolism mis-match when patients undergoing coronary recanalization also received intracoronary progenitor cells [77].
Stunned myocardium
Stunned myocardium has been defined as reversible myocardial contractile dysfunction in the presence of normal resting myocardial blood flow [73]. It is felt to represent a state of persistent myocardial contractile dysfunction that follows a period of transient ischemia (coronary occlusion), even after flow has been restored to an area that has no irreversible damage [74]. Stunning represents a flow-contraction mismatch that is typically seen in the clinical setting of thrombolytic therapy or angioplasty/stenting for acute myocardial infarction. In these cases, the region of affected myocardium will appear perfused on nuclear imaging exams, but will demonstrate wall motion abnormalities on gated imaging or echocardiography. Stunning represents the combined results of ischemic and reperfusion injury. In the classical setting (transient coronary artery occlusion), stunning may persist for a few days to 8 weeks after revascularization [1], but general improvement in regional wall motion is seen after 2 to 3 weeks without further intervention.
Stunning can also be seen in the recovery phase after exercise induced ischemia (i.e.: after stress testing, gated exams may show wall motion abnormalities in regions of ischemic myocardium that do not persist on rest images). The time course of resolution of ischemic LV dysfunction following exercise has been reported to range from immediate to up to 2 hours [47]. Stunning is also seen in the post-operative CABG period, in the setting of unstable angina, and following coronary angioplasty [6]. Stunning has been reported with pharmacologic stress imaging [44]. In these cases it is most likely due to a "coronary artery steal" in which the increased blood flow in a normal artery siphons flow away from the myocardial tissue distal to a high grade stenosis via collateral channels (i.e.: the affected segment becomes truly ischemic) [44].
Proposed mechanisms for the observed wall motion abnormalities include:
- Abnormal energy utilization by myofibrils
- Production of cytotoxic oxygen free radicals
- Altered calcium flux
- Accumulation of neutrophils in previously ischemic tissue
Hibernating myocardium
Hibernating myocardium represents viable myocardium with depressed resting flow and reduced resting function [64]. The decrease in oxygen delivery to the myocardium results in a down-regulation of mitochondrial oxidative metabolism and reduced contractile function [75]. Hence, there is a chronic reduction in myocardial metabolism/contractility in order to match a long-standing decrease in blood supply (chronic ischemia). In other words, the cells maintain viability, but cannot perform mechanical work [20]. This may be a protective response by the myocytes to reduce their oxygen demand in the setting of reduced oxygen availability. However, this is a reversible phenomenon and ventricular dysfunction can improve if flow is restored [64]. A broader definition of hibernating myocardium includes any myocardial region that will improve function after coronary revascularization [64]. Evidence suggests that myocardial hibernation is an incomplete adaptive response to ischemia which cannot be maintained indefinitely and that once identified, prompt revascularization should be performed as the condition can progress to irreversible injury [49,59,64,73]. Another theory proposes that hibernating myocardium is the result of repetitive episodes of myocardial stunning which leads to chronic left ventricular dysfunction [54]. The distinction between hibernation and chronic stunning can be based on the assessment of myocardial blood flow [54,88]. In hibernation resting blood flow is reduced, whereas the presence of normal resting blood flow, but decreased flow reserve suggests chronic stunning [54,88].
The presence of hibernating myocardium in a patient with LV dysfunction indicates a high risk for subsequent cardiac events when treated with medical therapy alone [49]. Because enhanced left ventricular function after revascularization is associated with improved survival, it is crucial to identify areas of viable myocardial tissue (hibernating myocardium).
The potential for improved function following revascularization is dependent upon many variables- of which the presence of viable myocardial tissue is of obvious importance. However, other factors such as the timing of revascularization after the onset of hibernation may influence the degree of recovery following cardiac surgery [52]. Hibernation is an adaptive response to ischemia and it does not last indefinitely [67]. In areas of hibernating myocardium there is progressive cellular degeneration over time which may reduce the chance for complete functional recovery if revascularization is delayed [52]. In fact, patients who undergo delayed revascularization following the identification of viable myocardium by FDG-PET imaging have a significant increased pre-operative mortality and minimal functional improvement [67]. Hence, once viable myocardial tissue is identified, prompt revascularization in indicated as delay can result in suboptimal outcome [67]. Other factors such as the presence and magnitude of stress-induced ischemia, the degree of left ventricular remodeling, and the adequacy of target coronary vessels can also all affect functional outcome [57]. In fact, LV volumes are very relevant for the determination of functional recovery and patients with severely dilated left ventricles have a low likelihood for showing improvement in LVEF [73]. In patients with substantial viability (equal to or more than 4 segments), a LV end-systolic volume of less than 130 mL had better survival rates compared to patients with volumes of greater than 130 mL [73].
On gated SPECT imaging or echocardiography areas of hibernating myocardium can be expected to demonstrate a wall motion abnormality.
Endpoints in Viability Studies:
Various criteria have been used to determine the clinical impact of revascularization of viable (hibernating) myocardial tissue including- 1- improvement in regional LV function (segments); 2- improvement in global LV function (LVEF); 3- improvement in symptoms (NY Heart Association functional class); 4- improvement in exercise capacity; 5- reverse LV remodeling; and 6- long term prognosis [73]. In general, a post-operative increase in LVEF is related to the amount of residual viable tissue [73] and improvement in global LV function (LVEF) is clinically more meaningful than improvement in segemental function [88]. The available evidence suggests that 20-30% (25%) of the LV needs to be viable to allow improvement in the LVEF [73,88]. Similarly, patients with extensive viability have been shown to demonstrate a significant improvement in exercise capacity [73]. These same patients (residual viability over 20% of the LV) have also been shown to demonstrate reverse LV remodeling with significant reductions in LV end-systolic and end-diastolic volumes following surgery [73]. Finally, in a meta-analysis of various viability techniques, the annual death rate in patients with viable myocardium that underwent revascularization was 3.2%,compared to 16% in patients treated medically [73].
Viability techniques:
The various methods available to determine myocardial viability include 18F-FDG PET imaging, SPECT imaging (using 201Tl or less commonly T-99m agents), echocardiography with dobutamine (to assess contractile reserve), MRI with dobutamine, and MRI with IV contrast (to assess scar) [73].
See also FDG PET imaging for myocardial viabiliy
1- Thallium imaging in the identification of hibernating myocardium:
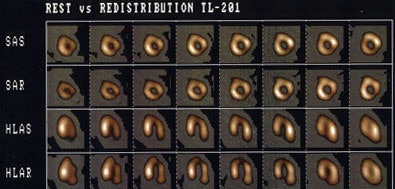
Rest-redistribution thallium imaging is used to assess for the presence of viable myocardial tissue with severely diminished perfusion (hibernating myocardium). A standard stress-rest-reinjection examination can provide similar information, as well as evaluate for the presence of ischemia. Stress-redistribution thallium or dual-isotope exams using a rest thallium injection may not be as useful, as between 40-50% of fixed thallium defects on non-reinjection images have been shown to be viable on FDG imaging.
Technique:
A rest-redistribution exam is useful when the clinical question pertains exclusively to the presence or absence of myocardial viability and not the detection of inducible ischemia. The exam is performed with a rest 3 to 4 mCi injection of thallium. Images should be acquired at the time of injection and 3 to 4 hours later. Reinjection prior to 4-hour delayed imaging is not performed and 24-hour delayed images are not generally required.
Findings:
Three types of abnormalities can be identified:
- Clearly reversible abnormalities: Rest perfusion defects which normalize on delayed images are indicative of severely ischemic, but viable myocardium (hibernating myocardium). Patients with areas of myocardial viability and left ventricular systolic dysfunction have been shown to have a much better prognosis when treated with surgical revascularization as opposed to medical therapy [36].
- Fixed defects with severely decreased tracer activity: Rest perfusion defects that do not demonstrate improved activity on delayed images most likely represent scar, although a small percentage (up to 20%) show improved regional wall motion following revascularization [37]. General, defects with less than 50% of peak activity are felt to represent scar (or more extensive non-transmural MI) with a low likelihood for improved LV function after revascularization. Note: For fixed defects with activity less than 50% of the maximal uptake, up to 25% have been shown to be viable by FDG imaging [38].
- Fixed defects with mild to moderate decreased tracer activity: About 50% of these regions show improved wall motion following revascularization. These areas may contain mixed viable myocardial tissue and scar (i.e.: regions of non-transmural myocardial infarction). A mild defect (less than 30% reduction in activity) is indicative of viability with a better likelihood for improvement in wall motion following revascularization. Other authors suggest that a fixed defect with counts of 50% or greater of the peak activity indicates myocardial viability, however, these areas frequently contain subendocardial scar tissue and may not improve in function following revascularization [73].
Sensitivity and specificity of rest thallium imaging:
Preserved thallium uptake in regions of myocardium with dysfunctional wall motion is predictive of functional recovery after revascularization. The sensitivity of rest-redistribution thallium for the determination of viability and improvement in wall motion is between 67% to 86%, and the specificity is between 55% to 77% [11,48,51,53,73]. The positive and negative predictive accuracies are 69-72% and 70-92%, respectively, for the determination of recovery of regional left ventricular dysfunction following revascularization [20,35]. The sensitivity and specificity for the exam will vary depending on what percentage of maximal uptake is used to determine viability [40]- generally, segments with greater than 50% of peak activity are considered viable. Quantitative analysis of rest-redistribution images can aid in exam interpretation. Overall, thallium imaging is felt to have a high sensitivity for the identification of viable myocardial tissue, but a lower specificity [51]. Because the positive predictive accuracy of the exam is generally lower, it may lead to unnecessary revascularization [48].
When compared to PET viability imaging, a large percentage of mild to moderate thallium defects (with over 50 to 65% of maximal activity) on rest-redistribution images have been confirmed to be metabolically active on PET studies. Unfortunately, rest-redistribution thallium imaging can underestimate viability. In severe defects with less than 50% maximal uptake, metabolic activity has still been identified in up to half of these areas on PET imaging [41]. Rest-redistribution thallium imaging is slightly less accurate than PET imaging (PET perfusion-metabolism imaging has a positive predictive value and negative predictive values of 76-83% and 84-92%, respectively [20, 35]).
Following revascularization, on average, LVEF improved from 30% to 38% in patients with viable myocardium on thallium imaging, compared to those without (the LVEF remained essentially unchanged 29% to 31%) [73]. Heart failure symptoms show significant improvement in patients with viable myocardium and there was improved long term survival following revascularization (cardiac death rate of 18% versus 41%) [73]. The amount of myocardium that needs to be considered viable for overall improved survival following revascularization may be as large as 39% (26% for FDG PET imaging) [83]. However, the decision to proceed with revascularization should be individualized as patient's with much smaller amounts of myocardial viability may show improvement in symptoms of refractory angina and improved survival due to reduced risk of arrhythmic death [83].
If there are no contraindications to stress testing a stress-rest-reinjection exam provides a more comprehensive assessment of the extent and severity of coronary artery disease (CAD) by demonstrating regional myocardial ischemia, without compromising information on myocardial viability [39].
Combined use of rest thallium imaging and dobutamine echocardiography (which evaluates contractile reserve) has also been evaluated for the assessment of myocardial viability [48,51,53]. The combined use of these exams may provide more accurate information than either technique independently [48,53], particularly in patients with an intermediate likelihood of viability by either test [51].
Reverse-Redistribution on rest-redistribution exams:
On rest-redistribution imaging two patterns of reverse-redistribution can be identified. In one pattern there is normal uptake on the rest image, but a defect is identified on the redistribution exam. This finding suggests a high grade stenosis in the vessel which supplies that segment. It is also frequently (up to 40% of cases) associated with impaired myocardial contractile function in the affected segment, but improvement in wall motion is very likely following revascularization.
In the other pattern, there is a defect which is present on the rest image, but the defect demonstrates significantly decreased activity on the redistribution exam. Wall motion defects in these regions are more common (up to 60%), typically severe, and recovery of wall motion abnormalities following revascularization is very unlikely. Segments which demonstrate this finding should be classified as regions of scarring [42,43].
2- Technetium agent imaging of hibernating myocardium and myocardial viability
Thallium imaging with reinjection is superior to Tc-Sestamibi (and likely Tc-tetrofosmin) imaging in identifying viable myocardium in patients with chronic coronary artery disease [7]. Since the primary determinant of technetium perfusion agent uptake is regional coronary blood flow rather than tissue viability, technetium agents can underestimate the presence of viable myocardium in states of greatly reduced perfusion [57]. Approximately 35 to 60% of reversible myocardial regions on Thallium reinjection imaging will appear as fixed defects on exercise-rest images with Tc-Sestamibi [7,8]. Even if defect severity is taken into account, Tc-Sestamibi studies are likely to prove ineffective in separating subendocardial infarction from hibernating myocardium [9].
Generally, the likelihood for the presence of viable myocardium on technetium imaging is determined by the severity of the fixed perfusion defect. A moderate reduction in activity (51 to 85% of normal) in a fixed defect is more suggestive of viable myocardium and improves concordance with thallium imaging. Uptake within a defect of over 55% of the maximum cardiac activity is associated with a 70% likelihood of improved regional wall motion after revascularization [46]. Regions with uptake less than 55% peak activity have a lower likelihood of demonstrating improved wall motion after revascularization [46]. Quantitative analysis has improved sensitivity for the detection of viability compared to visual analysis [50]. In fact, a substantial number of dysfunctional segments with no evidence of viability at visual analysis exhibit preserved tracer uptake at quantitative analysis and improved wall motion following revascularization [50].
In another study based upon comparison with PET FDG imaging, Tc-sestamibi uptake of less than 30% of the peak myocardial activity had a predictive value of over 80% that this area represented a scar [10]. Unfortunately, 25% of segments with defects of 41% of peak activity, and 50% of segments with defects of 60% of peak activity were shown to be viable on FDG imaging [10]. Therefore, rest perfusion sestamibi defects with an uptake between 40%-60% of peak activity are intermediate probability for functional improvement following revascularization and further evaluation may be needed to make an appropriate clinical decision in these cases [50]. In general, segments with severe Tc-sestamibi defects with preserved FDG uptake were associated with severe hypokinesis on wall motion studies [10].
Attempts to improve the detection of viable myocardial tissue
with technetium imaging agents include rest exams performed
following the administration of nitrates (most likely the result
of the vasodilatory effects of the nitrates with increased blood
flow and tracer delivery to areas supplied by diseased coronary
vessels) [11,12,56,66,79,86]. After nitrate administration, an
increase in regional sestamibi uptake of 10% or greater (to a
level of at least 55% of peak myocardial activity) predicts viable
myocardium and post-revascularization functional improvement
[58,79]. In one study, 40% of patients with apparent non-viable
segments on baseline rest imaging, were found to have viable
myocardium on the post-nitrate exam [79]. Patients with nitrate
viable myocardium also had a much higher cardiac event rate
compared to patients with non-viable tissue [79]. Although
concordance with FDG imaging has been shown to improve with the
use of nitrates [56,66], the results are still inferior to FDG PET
imaging [58]. Delayed redistribution cardiac imaging after resting
Tc-MIBI injection has also been investigated. Such delayed images
have revealed improved uptake of tracer in 24 to 38% of rest
defects [13,14] and better concordance with PET viability imaging
[56].
Post nitrate Tc-99m tetrofosmin has also been evaluated for the
determination of myocardial viability compared to FDG PET [86].
Using a cutoff for viable segments of >50% maximum tetrofosmin
uptake, attenuation corrected SPECT had an accuracy of 94%
(however, FDG imaging still identified additional viable segments
in 24% of patients and resulted in change in patient management in
13% of patients) [86]. One additonal benefit of technetium agent
imaging is the ability to measure LVEF- improvement in global or
regional LVEF following nitrate administration is considered a
strong indicator of improvement in LV function following
revascularization [86].
Despite these efforts to demonstrate the value of technetium agents, it must be remembered that reinjection thallium imaging (as discussed above) provides information regarding myocardial viability similar to that of PET imaging. The concordance between the two techniques regarding the presence of viable or nonviable myocardium is about 90%.
|
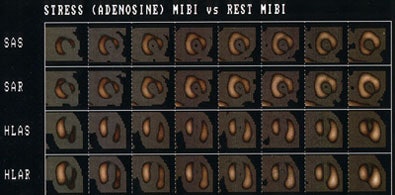
Tc-Sestamibi versus rest thallium for the detection of hibernating myocardium: The Tc-MIBI exam (top) demonstrates severe fixed perfusion defects involving the apex, anteroapex, and inferolateral wall. Because the patient had a severely reduced ejection fraction and was a candidate for revascularization, a rest thallium exam was performed to assess for the presence of viable myocardial tissue. The thallium exam (below) reveals extensive viable myocardial tissue in the regions of fixed Tc-MIBI defects. Thallium is superior to technetium agents for the assessment of myocardial viability. |
|
|
3- MR Imaging for Hibernating Myocardium:
MR imaging is also being studied for the assessment of myocardial viability [34]. Because of the excellent anatomic resolution that can be achieved with rapid cardiac imaging sequences, MR determination of viability is based on visible changes in myocardial wall thickness [34] or through the use of delayed contrast enhanced imaging. An advantage of delayed contrast enhanced MR is that it can detect subendocardial scar with greater sensitivity than SPECT [82]. However, one limitation of MR for viability is that it cannot distinguish hibernating myocardium from normally perfused myocardium in regions of nontransmural hyperenhancement [82].
Funcational myocardial evaluation:
For functional MR imaging, six to eight short axis sections can be obtained and images reviewed in cine format [34]. Hypokinetic or akinetic segments demonstrate less than 1 mm of wall thickening [34]. Normal myocardial tissue has an end-diastolic wall thickness greater than 5.5 mm and systolic wall thickening greater than 1.5 mm [34]. Hibernating myocardium has an end-diastolic wall thickens greater than 5.5 mm and wall thickening less than 1.5 mm [34]. Infarcted myocardium has an end-diastolic wall thickness less than 5.5 mm and wall thickening less than 1.5 mm [34]. Segments with an end-diastolic wall thickness of less than 5.5mm almost never show recovery of function after revascularization [73,88]. However, segments with a wall thickness of greater than 5.5 mm do not always show improved function following revascularization - possibly related to the presence of non-transmural infacrtion [73]. It should also be noted that even in the presence of severe wall thinning, recovery of function may occur, but only when contrast-enhanced MRI excludes scar [73]. Overall, wall thinning can be used to predict functional recovery following revascularization with a sensitivity of between 55-95% and a specificity of 41% [34,73]. Segments felt to be representative of hibernating myocardium that failed to demonstrate improved function were commonly adjacent to infarcted segments or had nontransmural wall thinning [34]. The lack of improved function in regions adjacent to areas of infarcted myocardium may be related to mechanical tethering [34]. Also, myocardium adjacent to areas of chronic infarction have hypertrophied myocytes that have impaired contractile function [34]. In regions where wall thickness is greater than 5.5 mm, but less than normal, there is a reduction in muscle mass and the amount of recovery can be reduced. A subendocardial infarct with some preserved end diastolic wall thickening (EDWT) may limit improvement in left ventricular function [34].
Patients with acute myocardial infarctions less than 3 weeks old should not be evaluated for viability as areas of stunned myocardium may not have regained full function and the areas of infarcted myocardium may not have had time to allow the scarring process to produce wall thinning [34].
Contrast enhanced imaging evaluation:
Because gadolinium is retained by scar tissue there is faster
washout of the contrast from the normal myocardium and delayed
contrast enhanced MR (DE MR) imaging is very sensitive for
detecting areas of myocardial infarction and scar. A major benefit
of MR imaging is its much higher spatial resolution permitting the
detection of even small areas of subendocardial infarction that
will be missed on SPECT imaging [76]. Remember- delayed contrast
enhanced MR imaging is specific for either acute MI or scar, but
not for hibernating myocardium or repetitively stunned myocardium
[76,80]. Delayed contrast enhanced MR can provide better
evaluation of the subendocardium for viability compared to FDG PET
imaging [87]. In one study, 11% of segments delineated as viable
by PET showed some late gadolinium enhancement and this was
particularly located in the subendocardium [87].
Delayed hyperenhancement is defined as regions of increased signal intensity on T1-weighted images acquired more than 5 minutes after IV gadolinium administration [73] and reflects irreversibly injured myocardium or replacement fibrosis [71]. DE MR has been shown to be superior to SPECT imaging for the detection of acute MI- particularly for inferior infarcts [61]. For the MR exam delayed inversion-recovery fast low-angle shot contrast-enhanced imaging is performed in the short axis plane 15 minutes following the IV administration of 0.15 mmol of gadolinium per kg of body weight [60]. Because Gd-DTPA concentration will change in the normal myocardium over time, the inversion time (TI) selected for imaging may require adjustment to avoid underestimation of infarct size if later imaging is performed [76]. The proper TI can be selected by acquiring a series of images after the inversion prepulse using a central short axis slice [76]. Visual or ROI analysis can be performed to select the TI that produces the lowest signal from normal myocardium [76].
Areas of myocardial infarction (both acute and chronic) demonstrate late contrast enhancement in these regions following the administration of gadolinium [60]. The phenomenon is most likely secondary to edema and an increase in the extracellular space at the site of infarction (scar) with delayed washout of contrast as a consequence of reduced capillary density [60,65,76]. Infarction always involves the subendocardium which is most vulnerable to ischemia [71]. Non-ischemic causes of delayed hyperenhancement should be suspected if the subendocardium is spared [71]. A central "dark" core can sometimes be observed within areas of hyperenhancement associated with acutely infarcted myocardium [76]. These areas of decreased signal are felt to be secondary to "no reflow" phenomenon which is defined histopathologically as evidence of microvascular damage and low myocardial perfusion [76]. The presence of this area of no reflow is associated with advanced myocardial damage and impaired functional recovery [76]. By about 30 days following the event, an area of infarction will demonstrate thinning of the myocardium and decreased signal on T2 images or gradient echo images due to the presence of fibrosis.
Because delayed enhancement can be seen in other cardiac conditions, it's presence must be interpreted in the appropriate clinical setting [70]. Other conditions associated with delayed enhancement include hypertrophic cardiomyopathy, dilated nonischemic cardiomyopathy, arrhythmogenic right ventricular dysplasia, myocarditis, sarcoid, amyloid, trauma, hypereosinophilic syndrome, and post radiofrequency ablation [70,71]. Unlike the pattern in myocardial infarction, delayed enhancement in these conditions is often in the mid wall of the myocardium (rather than subendocardial or transmural) and does not correspond to coronary vascular territories [70]. Transient subendocardial contrast enhancement has also been described in patients with syndrome X [70].
MRI in prognosis:
The transmural extent of hyperenhancement within the 1st week after MI can be used to predict late improvement in contractile function [60]. Lack of delayed enhancement on MR is highly predictive for functional improvement after surgical revascularization [72]. Regions with greater transmural enhancement are less likely to demonstrate improved wall motion (i.e: improvement in function decreases progressively as the amount of scar tissue increases) [60,72,73]. Segments with greater than 75% transmural extent of delayed hyperenhancement are very unlikely to recover function [65]. Only about 10% of segments demonstrating 51-75% hyperenhancement will demonstrate functional recovery with revascularization [65]. Segments with infarct involving less than 25% of the wall thickness are more likely to recover function following revascularization [63,76]. In fact, even in regions of akinesis or dyskinesis before revascularization, a lack of hyperenhancement is predictive of functional recovery [65]. However, the application of a threshold of transmurality for separating recovering from non-recovering myocardium has limited specificity [69]. When a transmural DE threshold of 50% is used the sensitivity for functional recovery is 92-95%, the specificity is 45%, the positive predictive value is 62-75%, and the negative predictive value is 79-92% [69,72,73,80]. Studies have consistently shown that the PPV of delayed enhancement is lower than the NPV [80]. Decreasing the threshold to 25% improves the PPV to 71%, but the NPV decreases to 79% [69]. Despite these data, MRI may result in non-diagnostic results in up to 36% of segments due to subjective interpretation of the extent of myocardial wall involvement [81]. FDG/perfusion imaging can provide an unimbiguous result in over 90% of patients [80]. Rather than a percentage estimate of the degree of late enhancement, a measurement of the amount of non-enhancing myocardium can also be used to assess for wall motion recovery [81]. A segment with >4.5 mm of non-enhancing myocardium correlates with greater than 50% FDG uptake on PET and will wall motion recovery [81].
Assessment of inotropic reserve using low dose dobutamine
infusion and wall motion analysis can be used to aid in predicting
functional recovery when the extent of myocardial delayed
enhancement is intermediate [76]. However, the sensitivity
decreases when the transmural extent of infarction is greater than
38% [76].
Dobutamine stress echocardiography:
R=The exam can be performed to evaluate areas of contractile
dysfunction [88]. During the stepwise infusion of dobutamine,
echocardiographic imaging is performed to assess regional wall
motion [88]. The hallmark of viability is contractile reserve
which is improvement of contactile dysfunction during low dose
dobutamine infusion (beginning at 5 to 10 mcg/kg/min and going up
to 40mcg/kg/min) [88]. The possible observed wall motion responses
during dobutamine infusion include (1) a biphasic response- with
initial improvement followed by worsening wall motion which
suggests viability with superimposed ischemia; (2) immediate
worsening with direct deterioration of wall motion without initial
improvement which suggests severe ischemia due to a critical
coronary stenosis; (3) sustained improvement without subsequent
deterioration which suggests subendocardial infarction; and (4) no
change which suggests transmural scar/infarct [88].
REFERENCES:
(1) J Nucl Med 1993; Schulman DS, et al. Right ventricular
thallium-201 kinetics in pulmonary hypertension: relation to right
ventricular size and function. 34: 1695-700
(2) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology (1). 329: 775-783
(3) Am J Cardiol 1990; Villanueva FS, et al. Prevalence and correlates of increased lung/heart ratio of thallium-201 during dipyridamole stress imaging for suspected coronary artery disease. 66: 1324-28
(4) Am.J.Cardiol 1990; Lette J, et al. Transient left ventricular cavitary dilation during dipyridamole-thallium imaging as an indicator of severe coronary artery disease. 66:1163-70
(5) J Nucl Med 1994; Iskandrian AS, et al. When is myocardial viability an important clinical issue? 35 (Suppl): 4S-7S
(6) J Nucl Med 1994; Leavitt JI, et al. Demonstration of viable, stunned myocardium with technetium-99m-sestamibi. 35: 1805-07
(7) J Nucl Med 1992; Cuocolo A, et al. Identification of viable myocardium in patients with chronic coronary artery disease: comparison of thallium-201 scintigraphy with reinjection and technetium-99m-methoxyisobutyl isonitrile. 33: 505-511
(8) Circulation 1994; Dilsizian V, et al. Myocardial viability in patients with chronic coronary artery disease. Comparison of 99mTc-sestamibi with thallium reinjection and [18F] fluorodeoxyglucose. 89: 578-87
(9) Radiol Clin North Am 1993; Kiat H, et al. Myocardial perfusion imaging using technetium-99m radiopharmaceuticals. 31: 795-815
(10) J Nucl Med 1994; Altehoefer C, et al. Significance of defect severity in technetium-99m-MIBI SPECT at rest to assess myocardial viability: comparison with fluorine-18-FDG PET. 35: 569-74
(11) J Nucl Med 1995; Maurea S, et al. Enhanced detection of viable myocardium by technetium-99m-MIBI imaging after nitrate administration in chronic coronary artery disease. 36: 1945-52
(12) J Nucl Med 1995; Bisi G, et al. Technetium-99m-sestamibi imaging with nitrate infusion to detect viable hibernating myocardium and predict postrevascularization recovery. 36: 1994-2000
(13) J Nucl Med 1995; Maurea S, et al. Myocardial viability index in chronic coronary artery disease: technetium-99m-methoxy isobutyl isonitrile redistribution. 36: 1953-60
(14) Circulation 1994; 89: 578-87
(15) J Nucl Med 1998; Bax JJ, et al. Comparison of Fluorine-18-FDG with rest-redistribution thallium SPECT to delineate viable myocardium and predict functional recovery after revascularization. 39: 1481-1486
(16) J Nucl Med 1995; Ohte N, et al. Clinical significance of
reverse redistribution on 24-hour delayed imaging of exercise
thallium-201 myocardial SPECT: comparison with myocardial
fluorine-18-FDG-PET imaging and left ventricular wall motion.
36: 86-92
(17) J Nucl Med 1993; Liu P, Burns RJ. Easy come, easy go: time to pause and put thallium reverse redistribution in perspective. 34: 1692-94
(18) J Nucl Med 1995; Soufer R, et al. Relationship between reverse redistribution on planar thallium scintigraphy and regional myocardial viability: a correlative PET study.36: 180-87
(19) Nucl Med Annual 1993; Parmett SR, Ongseng. Myocardial perfusion imaging: Pifalls and pearls. Ed. Freeman LM. Raven Press, NY. 195-221
(20) Radiographics 1999; Jadvar H, et al. SPECT and PET in the evaluation of coronary artery disease. 19: 915-926
(21) AJR 2000; Robinson VJB, et al. Causes of transient dilatation of the left ventricle during myocardial perfusion imaging. 174: 1349-1352
(22) Semin Nucl Med 1997; Kataoka T. False-positive myocardial perfusion scintigraphy in syndrome X. 27 (2): 186-189
(23) J Am Coll Cardiol 1996; Mazzanti M, et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilatation of the left ventricle in dual-isotope myocardial perfusion SPECT. 27: 1612-1620
(24) J Nucl Med 2000; Verna E, at al. "False-positive" myocardial perfusion scintigraphy findings in patients with angiographically normal coronary arteries: Insights from intravascular sonography studies. 41: 1935-1940
(25) J Nucl Med 2001; Bax JJ, et al. Relationship between preoperative vaiability and postoperative improvement in LVEF and heart failure symptoms. 42: 79-86
(26) J Nucl Med 2001; Tawakol A, Gewirtz H. Does CABG improve left ventricular ejection fraction in patients with ischemic cardiomyopathy, and does it matter? 42: 87-90 (No abstract available)
(27) J Nucl Med 2001; Matsumoto N, et al. Quantitative assessment of motion artifacts and validation of a new motion correction program for myocardial perfusion SPECT. 42: 687-694
(28) J Nucl Med 1993; Prigent FM, et al. Effect of motion on thallium-201 SPECT studies: a simulation and clinical study. 34:1845-50
(29) J Am Coll Cardiol 1997 Bax JJ, et al. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: Pooled comparison data. 30: 1451-60
(30) Semin Nucl Med 2000; Bas JJ, et al. 18-Fluorodeoxyglucose imaging with positron emission tomography and single photon emission computed tomography: Cardiac applications. 30: 281-298
(31) Semin Nucl Med 1995; Newhouse HK, Wexler JP. Myocardial perfusion imaging for evaluating interventions in coronary artery disease. 25: 15-27
(32) J Nucl Med 2001; Tanaka R, Nakamura T. Time course evaluation of myocardial perfusion after reperfusion therapy by 99mTc-tetrofosmin SPECT in patients with acute myocardial infarction. 42: 1351-1358
(33) J Nucl Med 2001; Harel F, et al. Clinical impact of combination of scatter, attenuation correction, and depth-dependent resolution recovery for 201Tl studies. 42: 1451-56
(34) Radiology 2001; Oshinski JN, et al. Quantitative prediction of improvement in cardiac function after revascularization with MR imaging and modeling: Initial results. 221: 515-522
(35) J Nucl Med 1994; Maddahi J. Role of thallium-201 and PET imaging in evaluation of myocardial viability and management of patients with coronary artery disease and left ventricular dysfunction. 35: 707-15
(36) Chest 1999; Morse RW, et al. Rest-redistribution 201-Tl single-photon emission CT imaging for determination of myocardial viability. 115: 1621-1626
(37) Circulation 1993; Ragosta M, et al. Quantitative planar rest-redistribution 201Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary bypass surgery in patients with severely depressed left ventricular function. 87: 1630-41
(38) J Nucl Med 1995; Burt RW, et al. Direct comparison of fluorine-18-FDG SPECT, fluorine-18-FDG PET and rest thallium-201 SPECT for detection of myocardial viability. 36: 176-79
(39) Circulation 1993; Dilsizian V, Bonow RO. Current diagnostic techniques of assessing myocardial viability in patients with hibernating and stunned myocardium. 87: 1-20
(40) (7) J Nucl Med 1998; Pace L, et al. Identification of viable myocardium in patients with chronic coronary artery disease using rest-redistribution thallium-201 tomography: Optimal image analysis. 39: 1869-1874
(41) J Nucl Med 1994; Hendel RC. Single-photon perfusion imaging for the assessment of myocardial viability. 35:(Suppl.): 23S-31S
(42) J Nucl Med 1993; Pace L, et al. Reverse redistribution in resting thallium-201 myocardial scintigraphy in patients with coronary artery disease: relation to coronary anatomy and ventricular function. 34: 1688-92
(43) J Nucl Med 1995; Pace L, et al. Reverse redistribution in resting thallium-201 myocardial scintigraphy in chronic coronary artery disease: an index of myocardial viability. 36: 1968-73
(44) J Nucl Cardiol 2001; Dakik HA, Alam S. Myocardial stunning induced and detected by adenosine stress perfusion imaging. 8: 711-2 (No abstract available)
(45) J Nucl Med 2002; Sciagra R, et al. Comparison of dobutamine echocardiography and 99mTc-sestamibi tomography for prediction of left ventricular outcome after acute myocardial infarction treated with successful primary coronary angioplasty. 43: 8-14
(46) J Nucl Cardiol 2002; Acampa W, et al. Tetrofosmin imaging in the detection of myocardial viability in patients with previous myocardial infacrtion: comparison with sestamibi and Tl-201 scintigraphy. 9: 33-40
(47) J Nucl Med 2002; Yamagishi H, et al. Incremental value of left ventriuclar ejection fraction for detection of multivessel coronary artery disease in exercise 201Tl gated myocardial perfusion imaging. 43: 131-139
(48) J Nucl Cardiol 2002; Dellegrottaglie S, et al. Prediction of the long term effects of revascularization on regional and global left ventricular function by dobutamine echocardiography and rest Tl-201 imaging alone and in combination in patients with chronic coronary artery disease. 9: 174-82
(49) J Nucl Cardiol 2002; Di Carli MF. Assessment of myocardial viability after myocardial infarction. 9: 229-235
(50) J Nucl Cardiol 2002; Acampa W, et al. Quantification of SPECT myocardial perfusion imaging. 9: 338-42
(51) J Nucl Med 2002; Bax JJ, et al. Sequential 201Tl imaging and dobutamine echocardiography to enhance accuracy of predicting improved left ventricular ejection fraction after revascularization. 43: 795-802
(52) J Nucl Med 2002; Matsunari I, et al. Sequential strategy using multimodality viability tests: does it work? 43: 803-805
(53) J Nucl Cardiol 2003; Piscione F, et al. Relationship between contractile reserve, Tl-201 uptake, and collateral angiographic circulation in collateral-deoendent myocardium: implications regarding the evaluation of myocardial viability. 10: 17-27
(54) J Nucl Cardiol 2003; Nowak B, et al. Assessment of myocardial viability in dysfunctional myocardium by resting myocardial blood flow determined with oxygen 15 water PET. 10: 34-45
(55) J Nucl Cardiol 2003; Beller GA. Clinical value of myocardial perfusion imaging in coronary artery disease. 10: 529-542
(56) J Nucl Cardiol 2003; He W, et al. Tc-99m tetrofosmin tomography after nitrate administration in patients with ischemic left ventricular dysfunction: relation to metabolic imaging by PET. 10: 599-606
(57) J Nucl Cardiol 2003; Di Carli MF. The quest for myocardial viability: is there a role for nitrate-enhanced imaging? 10: 696-699
(58) J Nucl Cardiol 2004; Giorgetti A, et al. Baseline/postnitrate Tc-99m tetrofosmin mismatch for the assessment of myocardial viability in patients with severe left ventricular dysfunction: comparison with baseline Tc-99m testrofosmin scintigraphy/FDG PET imaging. 11: 142-51
(59) J Nucl Cardiol 2004; Udelson JE, et al. The historical and conceptual evolution of radionuclide assessment of myocardial viability. 11: 318-334
(60) Radiology 2003; Kitagawa K, et al. Acute myocardial infarction: myocardial viability assessment in patients early thereafter- comparison of contrast-enhanced MT imaging with resting 201Tl SPECT. 226: 138-144
(61) Radiology 2004; Lund GK, et al. Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. 232: 49-57
(62) J Nucl Cardiol 2004; Maddahi J. Factors influencing predictive value of FDG imaging for evaluating myocardial viability. 11: 526-526
(63) Circulation 2001; Choi KM, et al. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. 104: 1101-1107
(64) J Nucl Med 2005; Canty JM, Fallavollita JA. Hibernating myocardium. 12: 104-119
(65) J Nucl Cardiol 2005; Isbell DC, Kramer CM. Cardiovascular magnetic resonance: structure, function, perfusion, and viability. 12: 324-336
(66) J Nucl Med 2005; Giorgetti A, et al. Baseline/postnitrate tetrofosmin SPECT for myocardial viability assessment in patients with postischemic severe left ventricular dysfunction: new evidence from MRI. 46: 1285-1293
(67) Radiol Clin N Am 2005; Takalkar A, et al. PET in cardiology. 43: 107-119
(68) J Nucl Med 2006; Beeres SLMA, et al. Effect of intramyocardial injection of autologous bone marrow-derived mononuclear cells on perfusion, function, and viability in patients with drug-refractory chronic ischemia. 47: 574-580
(69) J Nucl Cardiol 2006; Bengel FM. Positron emission tomography and magnetic resonance imaging in heart failure. 13: 145-149
(70) J Nucl Cardiol 2007; Dilsizian V. Cardiac magnetic resonance versus SPECT: are all noninfarct myocardial regions created equal? 14: 9-14
(71) AJR 2007; Lim RP, et al. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. 188: 1675-1681
(72) J Nucl Med 2007; Wu YW, et al. Comparison of contrast-enhanced MRI with 18F-FDG PET/201Tl SPECT in dysfunctional myocardium: relation to early functional outcome after surgical revascularization in chronic ischemic heart disease. 48: 1096-1103
(73) J Nucl Med 2007; Schinkel AFL, et al. Assessment of myocardial viability in patients with heart failure. 48: 1135-1146
(74) Radiology 2007; Wu JC, et al. Cardiovascular molecular imaging. 244: 337-355
(75) J Nucl Cardiol 2007; Dilsizian V. Metabolic adaptation to myocardial ischemia: the role of fatty acid imaging. 14: S97-99
(76) J Nucl Cardiol 2008; Saraste A, et al. Contrast-enhanced magnetic resonance imaging in the assessment of myocardial infarction and viability. 15: 105-117
(77) J Nucl Med 2008; Kendziorra K, et al. Effect of progenitor cells on myocardial perfusion and metabolism in patients after recanalization of a chronically occluded coronary artery. 49: 557-563
(78) J Nucl Cardiol 2008; Kaminek M, et al. Individual differences in the effectiveness of intracoronary bone marrow cell transplantation assessed by gated sestamibi SPECT/FDG PET imaging. 15: 392-399
(79) J Nucl Cardiol 2009; Evangelista L, et al. Incremental prognostic value of cardiac single-photon emission computed tomography after nitrate administration in patients with ischemic left ventricular dysfunction. 16: 38-44
(80) J Nucl Cardiol 2010; Maddahi J. Viability assessment with MRI is superior to FDG-PET for viability: pro. 17: 292-297
(81) J Nucl Cardiol 2010; Patterson RE, et al. Viability assessment with MRI is superior to FDG-PET for viability: con. 17: 298-309
(82) J Nucl Cardiol 2010; Beller GA. The case for cardiac magnetic resonance and positron emission tomography multimodality imaging of myocardial viability. 17: 527-528
(83) J Nucl Cardiol 2010; Inaba Y, et al. Quanity of viable
myocardium required to improve survival with revascularization in
patients with ischemic cardiomyopathy: a meta-analysis. 17:
646-654
(84) J Am Coll Cardiol 2002; Allman KC, et al. Myocardial
viability testing and impact of revascularization on prognosis in
patients with coronary artery disease and lfet ventricular
dysfunction. 39: 1151-1158
(85) N Engl J Med 2011; Bonow RO, et al. Myocardial viability and
survival in ischemic left veicular dysfunction. 364: 1617-1625
(86) J Nucl Cardiol 2012; Raja S, et al. Comparison of nitrate
augmented Tc-99m tetrofosmin gated SPECT imaging with FDG PET
imaging for the assessment of myocardial viability in patients
with severe left ventricular dysfunction. 19: 1176-1181
(87) J Nucl Cardiol 2013; Ademaw N, Salerno M. PET/MRI: current
state of the art and future potential for cardiovascular
applications. 20: 976-989
(88) J NUcl Cardiol 2015; Bax JJ, Delgado V. Myocardial viability
as integral part of the diagnostic and therapeutic approach to
ischemic heart failure. 22: 229-245