Women's imaging firm Hologic has received the CE Mark for its Localizer wireless radiofrequency identification breast lesion localization device.
Clinicians can implant Hologic's Localizer tag inside a patient's breast up to 30 days before surgery and use a handheld reader to pinpoint the precise location of breast lesions prior to their removal. The Localizer device is designed to replace the traditional wire-guided method for localizing breast lesions, allowing for improved comfort and convenience for patients and clinicians, according to Hologic.
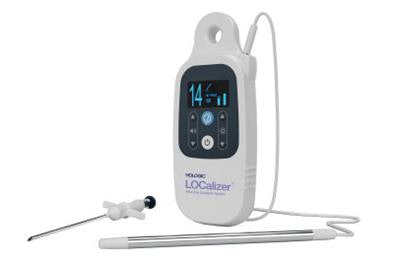 The Localizer wireless breast lesion localization device. Image courtesy of Hologic.
The Localizer wireless breast lesion localization device. Image courtesy of Hologic.The device is on display at the Hologic booth at ECR 2019 in Vienna, along with the company's entire suite of breast and skeletal health products.