A clinical trial comparing the effectiveness of two doses of a chemotherapy drug combined with radiotherapy showed equivocal results for patients with glioblastoma. However, the research did prove the feasibility of collecting and analyzing tumor tissue prospectively in an international, multicenter setting, the Radiation Therapy Oncology Group (RTOG) announced.
RTOG 0525 was a phase III clinical trial comparing the effectiveness of conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. Both groups also received radiotherapy treatments.
Neither dose proved to be more effective than the other for the 1,173 patients enrolled in the trial. Study results will be formally presented next month in Chicago at the American Society of Clinical Oncology (ASCO) annual meeting.
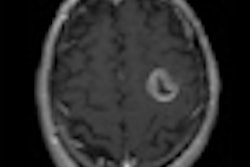
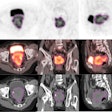
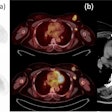
The study results confirmed the prognostic significance of MGMT (O6-methylguanine-DNA methyltransferase) gene methylation, according to RTOG principal investigator Dr. Mark Gilbert, professor of neuro-oncology at MD Anderson Cancer Center at the University of Texas. The results also demonstrated the feasibility of prospective tumor tissue collection, molecular stratification, and collection of patient outcomes in a large transatlantic intergroup trial, he said.
Dr. Kenneth Aldape, co-chair for neuropathology and correlative biology and professor of pathology at MD Anderson, added that the trial demonstrated the positive correlation between clinical outcomes and molecular classification of glioblastoma. He believes that it will improve future prognostic prediction, spur the development of specific therapies based on tumor biology, and, ultimately, lead to individualization of treatment.
RTOG 0525 was conducted in conjunction with the European Organization for Research and Treatment of Cancer (EORTC) and the North Central Cancer Treatment Group (NCCTG).
One hundred eight-five facilities in North America and 24 facilities in Europe participated. Key study eligibility criteria required that tumor tissue, obtained at the time of biopsy or surgery and with patient consent, be sent for central pathology review to confirm the tumor as a glioblastoma and as adequate to perform MGMT gene methylation analysis and molecular risk classification. This was done to assess whether a correlation existed between these biomarkers and study participant outcomes.