Chemotherapy delivered concurrently with radiation therapy (RT) is more effective than treatments delivered sequentially for extending the lives of patients with inoperable stage III non-small cell lung cancer (NSCLC), according to a study published online September 8 in the Journal of the National Cancer Institute.
The randomized clinical trial, Radiation Therapy Oncology Group (RTOG) 9410, aimed to confirm the importance of administering cisplatin-based chemotherapy concurrently with radiation therapy. Results showed modest but statistically significant survival outcome improvements: Patients receiving concurrent treatment lived a median of two months longer than patients who had sequential treatment, and more patients in the concurrent treatment group were alive five years later (JNCI, September 8, 2011).
The study included 610 patients with inoperable non-small cell lung cancer confined to the thorax, who were enrolled between July 1994 and July 1998 by 153 cancer treatment centers located in the U.S. and Canada. The patients had a median age of 61 years but were as young as 33 and as old as 80. The groups were statistically similar with respect to age, performance status, sex, histological subtype, and stage (II, IIIA, or IIIB).
Patients were randomly assigned to receive one of three treatments:
- Chemotherapy followed by radiation therapy
- Concurrently administered chemotherapy and daily radiation therapy
- Concurrently administered chemotherapy and twice-daily radiation therapy
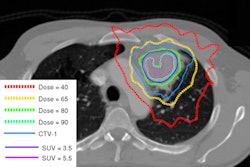
Radiotherapy began on day 50 for patients who had sequential treatment. They received a daily dose of 1.8 Gy to a total dose of 45 Gy over five weeks. During the sixth and seventh week, a daily dose of 2 Gy for nine fractions to 18 Gy was delivered to a smaller target volume encompassing the primary tumor and lymph nodes known to be involved with disease, as well as any lymph node larger than 2 cm.
Patients who received daily radiation therapy in conjunction with their chemotherapy began treatment on day 1 of the study. Radiotherapy treatment otherwise was identical.
Patients assigned to the hyperfractionated radiation therapy arm received twice-daily 20-Gy fractions to a total dose of 69.6 Gy separated with an interfractional time interval of six to eight hours. Target volume definitions were identical to those in the first two arms. The total dose was 50.4 Gy for the initial volume and 19.2 Gy for the secondary target volume. Treatment began on day 1, as did chemotherapy for this group.
Outcomes of 577 patients ultimately could be analyzed, with 195 patients in the sequential group, 195 in the concurrent daily radiotherapy group, and 187 in the hyperfractionated radiotherapy group. The median survival was longest for patients who received chemotherapy with daily radiotherapy: 17 months, compared with 15.6 months for the twice-daily radiotherapy group and 14.6 months for the sequential group.
When analyzing the percentage of patients alive five years following therapy, the highest percentage was found in the concurrent daily radiotherapy group, at 16%, followed by the twice-daily concurrent group at 13% and the sequential group at 10%.
"The significant increase in five-year survival for patients receiving concurrent versus sequential treatment establishes a new treatment standard for this large population of lung cancer patients," said Dr. Walter Curran Jr., the trial's principal investigator and executive director of the Winship Cancer Institute of Emory University. "It is likely that the chemotherapy makes the tumor cells more sensitive to radiation therapy, which contributed to the improved long-term patient benefit seen with concurrent therapy."
Dr. Jeffrey Bradley, chairman of the RTOG Lung Cancer Committee, added that "the significant survival benefit of concurrent treatment confirms the promising results observed in a similar RTOG phase II trial and corroborates the results of a 314-patient Japanese trial and several smaller European trials." Bradley heads the S. Lee Kling Center for Proton Therapy at the Washington University School of Medicine's Siteman Cancer Center.
Concurrent chemoradiotherapy did generate significantly worse acute toxicities than sequential treatment, the authors reported. The worst of these was severe esophagitis. Late side effects, however, were minimal and comparable among the three treatment groups.
RTOG is currently evaluating the addition of cetuximab, a monoclonal antibody that targets the epidermal growth factor receptor, to a concurrent chemotherapy and radiation therapy regimen in a phase III trial (RTOG 0617) of patients with stage III NSCLC. The study will help determine whether newer targeted therapies can improve upon the chemoradiation paradigm.