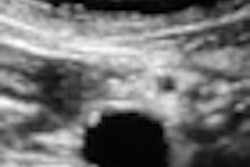
Ultrasound contrast developer Acusphere has signed a special protocol assessment agreement with the U.S. Food and Drug Administration (FDA) for the design of a new phase III study of its Imagify agent.
The study will compare stress ultrasound with Imagify to unenhanced stress ultrasound for detecting coronary artery disease, according to Acusphere. The FDA had requested a placebo-controlled phase III trial for Imagify to supplement earlier trials that compared Imagify's efficacy with nuclear stress testing.
Based upon an enrollment of 900 patients, the company expects the new trial will cost approximately $15 million and require approximately two years to complete.