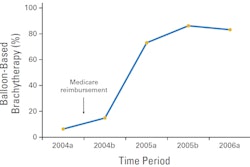
The U.S. Food and Drug Administration (FDA) on April 13 announced a recall of a breast shield marketed by iCAD for use during intraoperative radiation therapy (IORT) procedures with the company's Axxent product.
The FDA said that the Xoft Axxent FlexiShield Mini product, Model 5300, may shed particles of tungsten after IORT procedures. Although the agency said there is no evidence that the particles are toxic, they can be mistaken for suspicious calcifications during follow-up imaging procedures.
The device was originally developed by Xoft, which iCAD acquired in December 2010. Problems with the shields were described in a March 21, 2011, article by the New York Times, which reported that at least 11 women had tungsten residue in their breasts following Axxent procedures.
The FDA advised users of the model covered by the recall to stop using all units in their inventory and return them to the company. Healthcare professionals should inform patients about the likelihood of postoperative tungsten particles in the breast and continue the recommended follow-up protocol for the full five years, unless directed otherwise by the patient's treatment team.
The full text of the recall notice is available by clicking here.