VIENNA - U.S. National Institutes of Health (NIH) researchers, who spent years developing an advanced VC computer-aided-detection (CAD) scheme, recently revamped the polyp-detection algorithm to significantly improve its performance.
Research presented at today's virtual colonoscopy (VC or CT colonography [CTC]) sessions at the European Congress of Radiology (ECR) showed that the NIH system's ability to find medium-sized (6- to 9-mm) polyps was significantly improved by tweaking and simplifying the mathematical formula used to detect the shapes that define the morphology of colonic polyps.
As a result, radiologists performing VC may get a better evaluation of a class of colorectal polyps they need to keep tabs on -- though not necessarily remove unless they grow in size.
Available data on the natural history of polyps suggest that 10 mm is the threshold above which colonic polyps are at significantly greater risk of progressing to malignancy.
"I think most radiologists agree that polyps 10 mm and larger are important, but there's a sense that 6- to 9-mm polyps are in a size range that's important for clinical management," said Dr. Ronald Summers, of the National Institutes of Health in Bethesda, MD, in his presentation. "I've advocated that such polyps be left alone and observed by having surveillance CTC to see whether the polyp grows, since most of these polyps will not grow, and some may even shrink."
The CAD system works by preparing a 3D surface rendering of the colonic surface from prone and supine CT data entered into the system. The initial detection stage analyzes shape features along the surface of the colon to detect polyp candidates, Summers explained. Next is segmentation of those polyp candidates, in which the software tries to find the precise borders of each candidate in three dimensions.
This is followed by feature extraction and selection, wherein the borders are measured to determine the candidate lesion's size and shape. Finally a classifier, a type of machine learning algorithm, determines whether the CAD system considers the candidate a polyp or not.
The system was described in a 2005 study that yielded sensitivity of about 89% for polyps 10 mm or larger, but only about 52% for polyps in the 6- to 9-mm size range (Gastroenterology, December 2005, Vol. 129:6, pp. 1832-1844).
The new research, led by Summers' NIH colleague and postdoctoral research fellow Jiang Li, Ph.D., aimed to improve detection rates and streamline the mathematics.
In the 2005 study, "the sensitivity was 89% for polyps 10 mm or larger, but only about 52% for polyps in the 6- to 9-mm size range, and I wasn't satisfied with the performance level," Summers said.
Moreover, the initial polyp detection algorithm was needlessly complex. The heuristic method was based on a histogram of feature values that checked the mean and gaussian curvature of the polyp candidate, the average sphericity in clusters, and the number of vertices in the clusters -- then rechecked each one based on as many as 14 different thresholds to refine the initial detection of polyp candidates.
The new method is based on a multiobjective genetic algorithm that minimizes the number of false positives and false negatives simultaneously by means of multiple nondominated solutions known as a Pareto front, Summers said.
"The Pareto front shows points that are so-called nondominated. What that means is for any false-positive point along the x-axis, there was no set of parameters that led the CAD system to have a lower false-negative rate at that false-positive setting," Summers said. "So this greatly reduces the number of numerical values for these different thresholds, but we're still not done because any one of these points along the Pareto front could be the settings for our system."
The data were used to create free-response receiver operating characteristic (FROC) curves for three operation points on the Pareto front. Point 2 on the Pareto front was chosen for the system based on its ratio of true-positive to false-positive detections.
Computing the Pareto front for the initial training sets took several hours, even with the aid of a Biowulf supercomputing cluster, according to Summers. Daily clinical use of the system is much simpler, however; a prone and a supine CT dataset can be processed in about 10 minutes on a standard desktop PC.
The new algorithm was applied to 1,186 patients (700 men, 486 women) randomly partitioned into training (n = 394) and testing (n = 792) datasets at a 1:2 ratio. The data contained 201 medium-sized (6-to 9-mm) colonoscopy-confirmed polyps, including 132 adenomas with 41 adenomas in the training set and 91 adenomas in the testing set.
When the optimized system was tested on the training datasets, sensitivity was 69 ± 4% for medium-sized polyps, compared to 54 ± 3% for the old method, a statistically significant improvement (p <10-3). False-positive detections per patient were 8.1 versus 7.8 for the old system, a trend that did not reach statistical significance.
"This optimized CAD system has a significantly higher sensitivity for detecting medium-sized polyps -- that sensitivity increased by 15%," he said. "The sensitivity of this optimized CAD system closely approximates that of radiologists who did not have a CAD in reported series." That 15% included several medium-sized polyps that the old system had missed.
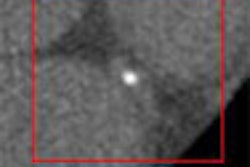
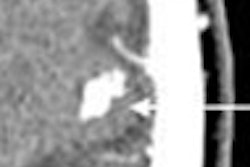
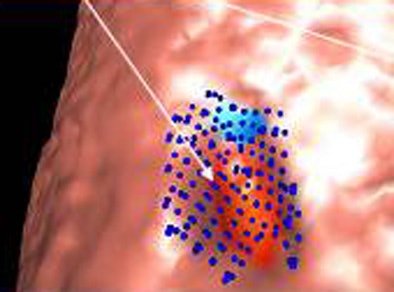 |
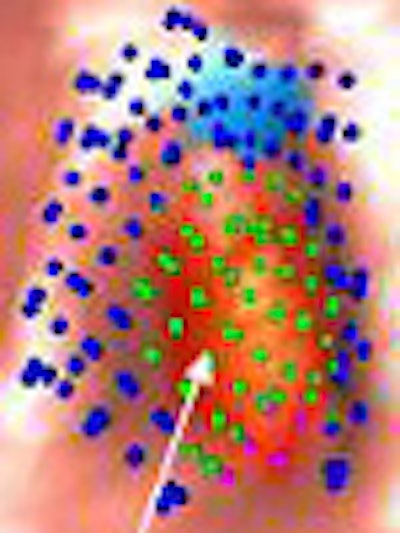
| Image (above) represents a medium-sized polyp that was initially missed by the CAD system, but detected (below) by the revamped algorithm. Blue dots signify points defined as ground truth, green dots detected vertices, and purple dots detected vertices not marked as ground truth. All images courtesy of Dr. Ronald Summers. |
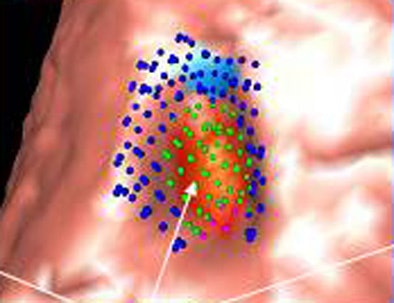 |
Sensitivity is slightly lower when hyperplastic polyps are included, for reasons that are unknown, Summers said in response to a question from the audience.
"Adenomatous polyps are slightly easier for the CAD to detect, but that's fortunate because the adenomatous polyps are the ones with the premalignant potential," he said.
Summers said he believes that hyperplastic polyps, because they are less firm, may flatten out a bit during colonic insufflation, and flat polyps are harder for the CAD to detect. "That's my working hypothesis," he said.
Summers said he still recommends that CAD be limited to use as second reader, although he is interested in research documenting the performance characteristics for CAD as a first or concurrent reader.
By Eric Barnes
Auntminnie.com staff writer
March 12, 2007
Related Reading
VC CAD delivers accuracy on unfamiliar datasets, February 5, 2007
CAD will be key to VC screening, September 13, 2006
VC visualization tool combines advanced features, August 17, 2006
VC CAD found equivalent to colonoscopy in screening population, December 21, 2005
Support vector machines boost accuracy of VC CAD, May 3, 2005
Copyright © 2007 AuntMinnie.com