In dealing with the onslaught of pulmonary nodules brought about by multidetector-row CT, radiologists are finding they need to hone their management strategies or risk being inundated. The goal is to maximize the chance of finding early-stage lung cancers, but minimize the risks, radiation, and wasted patient care that come from being too aggressive.
In a talk at the 2006 International Symposium on Multidetector-Row CT in San Francisco, Dr. Ann Leung, associate chairman of Stanford Radiology in Stanford, CA, suggested an approach based not only on established size and risk guidelines, but on individual patient factors and the morphology of the nodule at hand. It's a fine line to walk, of course, but in the end the best decision is the one the radiologist can be comfortable with, according to Leung.
Meanwhile, a study in the New England Journal of Medicine suggests that gene-expression profiles could potentially improve the management of early-stage lung cancer patients.
Management of nodules
"Based on screening studies, it's been recognized that over 50% of patients may have these noncalcified pulmonary nodules less than 8 mm in size," Leung said. "As we're all aware, the radiologic standard for determining benignity for these indeterminate nodules is two years' stability. But in fact, we recognize that the vast majority of these lesions will be benign. The question now posed to us is, are serial studies necessary in all patients, and if not what is a reasonable approach to management?"
What are the guidelines? A 2005 paper by the Fleischner Society makes a good framework, Leung said (Radiology, November 2005, Vol. 237:2, pp. 395-400).
The society's recommendations are divided into low- and high-risk patients, and by nodule size at detection, with additional caveats:
Low risk (patients 35 years or younger with minimal or absent risk of smoking and/or other risk factors)
- ≤ 4 mm, no follow-up needed
- 4-6 mm, follow-up CT at 12 months; if unchanged no further follow-up
- 6-8 mm, initial follow-up CT at six to 12 months, then at 18 to 24 months if no change
- 8 mm, follow-up CT at around three, nine, and 24 months, dynamic contrast-enhanced CT, PET and/or biopsy
High risk (patients older than 35 with a history of smoking or other risk factors)
- ≤ 4 mm, follow-up CT at 12 months; if unchanged no further follow-up
- 4-6 mm, initial follow-up CT at six to 12 months, then at 18-24 months if unchanged
- 6-8 mm, initial follow-up CT at three to six months, then at nine to 12 and 24 months if unchanged
- 8 mm, same as low-risk patient
Nonsolid (ground-glass) or partly solid nodules may require longer follow-up to exclude indolent adenocarcinoma, the authors added.
"With regard to the Fleischner guidelines ... we have to recognize that this is a consensus statement that was developed by a society of academic physicians with a major interest in chest disease," Leung said. "The management they propose is based on size, morphology, and clinical risk factors. They also clearly state in this consensus that these recommendations are likely to evolve as we gain more experience with small pulmonary nodules."
That said, not all of the group's recommendations are based on clinical experience, Leung said.
"I will say that scientific data support only their first recommendation, which is that nodules less than or equal to 4 mm in size in the lowest-risk group probably should have no follow-up, whereas those same nodules in the high-risk group requires 12-month follow-up," she said. The conclusion is supported by screening studies, which have never encountered a nodule 4 mm or smaller that has been diagnosed as lung cancer at follow-up 12 months or less, Leung said.
Starting from there
Some implicit boundary conditions must be recognized when using the guidelines, Leung said. For example, they don't apply to patients with known prior malignancies because the biology of metastases is quite different from that of primary bronchogenic carcinoma.
Also, "interval growth requires further evaluation or intervention at the time of detection of that growth," Leung noted. Partly solid or nonsolid tumors may require follow-up beyond two years in recognition of the fact that nodules with ground-glass components -- typically adenocarcinomas, possibly bronchoalveolar cell carcinomas -- have a much longer doubling time than more aggressive adenocarcinomas. Finally, conservative therapy is appropriate for elderly patients or those with major co-morbid diseases, she said.
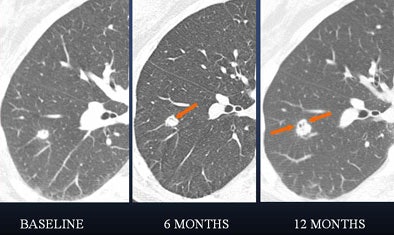 |
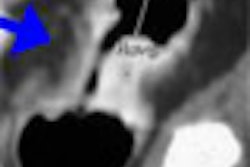
| In a 61-year-old woman a 6-mm nodule was found in the right lower lobe at baseline CT scan. At six-month follow-up, the nodule was determined to be stable in size. At 12 months, however, it had clearly grown in size. The nodule was biopsied and eventually diagnosed as adenocarcinoma. All images courtesy of Dr. Ann Leung. |
"If you look at the 12-month study (61-year-old woman, above), you will appreciate air lucency within that nodule," Leung said. "We're recognizing now as we are scrutinizing nodules much more carefully that the presence of air bronchograms is a concern for malignancy," she said. "If we look back at the first image, at six months, we can see that this lesion also had air bronchogram. We should have been more aggressive in trying to provide a diagnosis for this patient."
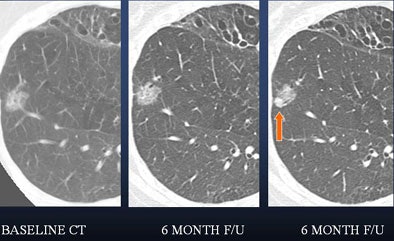 |
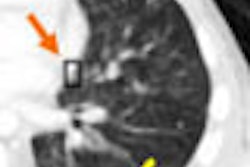
| In a 50-year-old male nonsmoker, a ground-glass nodule is detected in the right lower lobe. At six-month follow-up a solid component could be seen along the inferior aspect of the nodule, suggesting a heightened risk of malignancy. At this point, continued follow-up was deemed too risky, and the lesion was biopsied. The diagnosis was bronchoalveolar cell carcinoma (BAC). |
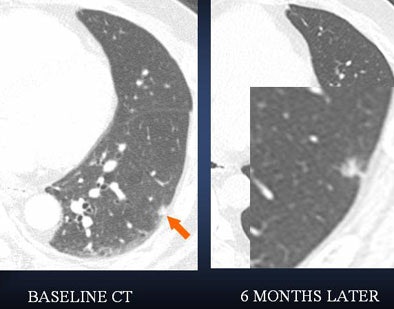 |
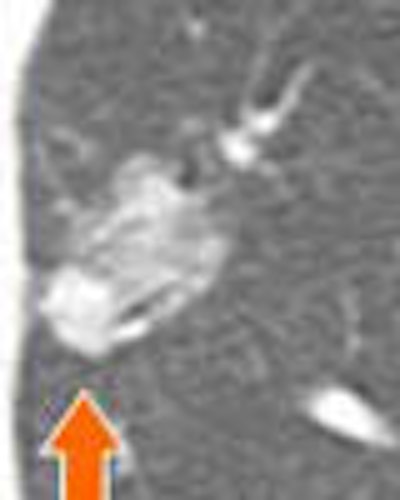
| A lung nodule detected in a 48-year-old man demonstrates highly irregular borders at six-month follow-up. PET could be helpful as a next step -- positive results would indicate more aggressive management. |
"Small indeterminate pulmonary nodules are common and most likely benign," Leung concluded. "Management requires triage based on size, clinical risk factors, and CT morphology. And I think the Fleischner guidelines should be tailored to individual practices, individual cases, and individual radiologists' comfort level with the possibility of misdiagnoses."
Genetic profiles could manage treatment
In lung cancer treatment news, a study in the New England Journal of Medicine used a genetic model to assess patients with stage IA lung cancer. The researchers hoped to select a subgroup of patients that would respond better to chemotherapy, noting that the stage classification scheme is probably an imprecise predictor of prognosis. In contrast to other early-stage lung cancers, studies have not shown a chemotherapy benefit in a stage IA patient cohort (NEJM, August 10, 2006, Vol. 355:6, pp. 570-580).
The researchers identified gene-expression profiles to predict the risk of recurrence in patients with non-small cell lung cancer (NSCLC), and evaluated their model in 134 patients. In two groups of patients, the lung metagene model had an overall predictive accuracy of 72% and 79%, respectively. The predictor also identified a subgroup of stage IA patients at high risk for recurrence and who might be best treated by adjuvant chemotherapy.
The model predicted recurrence for individual patients significantly better than did clinical prognostic factors, and was consistent across all early stages of NSCLC, the team concluded. The metagene model "provides a potential mechanism to refine the estimation of a patient's risk of disease recurrence and, in principle, to alter decisions regarding the use of adjuvant chemotherapy in early-stage NSCLC," they wrote.
By Eric Barnes
AuntMinnie.com staff writer
August 16, 2006
Related Reading
Canadian lung CAD cuts false positives, July 24, 2006
CAD improves lung nodule detection, June 1, 2006
Management strategies evolve as CT finds more lung nodules, August 19, 2005
Copyright © 2006 AuntMinnie.com