A new multicenter trial under way in the U.S. aims to assess VC's efficacy as a screening tool compared with conventional colonoscopy, using an up-to-the-minute VC protocol.
Researchers for the ACRIN National CT Colonography Trial (American College of Radiology Imaging Network protocol No. 6664) say they expect to enroll more than 2,300 patients at 15 sites nationwide over the next year. Screening is open to men and women age 50 and older who have not had a colonoscopy in the past five years, are scheduled for conventional colonoscopy, and have been referred for the trial by a primary care physician.
Investigators hope the ACRIN trial will provide the definitive word on CT colonography's current capabilities as a colorectal cancer screening tool. Imaging is already under way at two sites, with several more expected to begin screening this week.
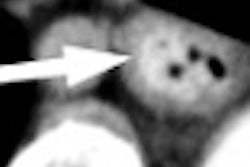
"The main outcome obviously is sensitivity for polyps 10 mm and greater, but there's going to be five or six or seven other publications that come out," said Dr. Richard Obregon, study participant and radiologist from Radiology Imaging Associates in Denver, who spoke with AuntMinnie.com by telephone on Monday.
Later studies will use the data obtained during initial screening to evaluate bowel-cleansing techniques, the efficacy of primary 2D versus 3D reading, the sensitivity of various CAD schemes, and eventually, the cost effectiveness and outcomes of screening using virtual versus conventional colonoscopy.
"The idea is to provide a more acceptable and high-performance examination that will translate into more patients undergoing the test, and consequently a reduction in overall colon cancer mortality," principal investigator Dr. C. Daniel Johnson from the Mayo Clinic in Rochester, MN, told the Reston, VA-based American College of Radiology. "Preliminary study results suggest that CT colonography (VC) is highly competitive with other screening tests. However, prior to its widespread clinical use, the test's accuracy must be evaluated in a large, multicenter trial that compares the latest CT colonography technology with the 'gold standard' of colonoscopy," Johnson said.
Nuts and bolts
According to Obregon, nearly all study participants will undergo same-day conventional and virtual colonoscopy, using whatever prep the endoscopist chooses for conventional colonoscopy. This means that some patients will have "wetter" preps such as those formulated with polyethylene glycol, while others, presumably those without significant comorbidities, will more often receive "drier" magnesium citrate-based preps that leave less residual fluid, and are consequently favored for VC.
Fortunately, fecal and fluid tagging will also be included. According to Obregon, participants will ingest a barium preparation (Tagitol V) and NutraPrep low-residue meal kit (both from E-Z-EM, Lake Success, NY) for two days before imaging, along with two doses (5 cc each) of an iodinated oral contrast agent (diatrizoate meglumine and diatrizoate sodium: MD Gastroview, Mallinckrodt, St. Louis, MO; or Gastrografin, Schering, Berlin).
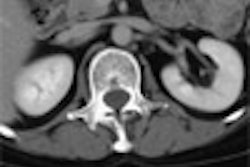
All CT scanners will be equipped with a minimum of 16 detector rows, and a few sites have installed 64-slice machines, Obregon said. Collimation will be set at 1.0-1.25 mm, with mAs and kVp at approximately 50 and 100, respectively. For interpretation, 1.5-mm-thick slabs with 50% overlapping reconstruction will be used, he said.
The participating radiologists, who have undergone extensive VC training and testing as a prerequisite for the study, will be randomized to perform all of their readings using either primary 2D with 3D for problem solving -- or alternatively, primary 3D with 2D problem solving, Obregon said.
"What happens is that the same study electronically gets sent to ACRIN, and ACRIN will then send it out to a virtual practice partner, who will read the study in the opposite manner." This will provide extensive data for comparing 2D versus 3D primary reading, he said.
There is no stipulation as to the manufacturer of the interpretation software, Obregon said, other than that the application should be capable of providing a primary 3D read in 10-12 minutes.
That capability that is now available on software packages produced by various manufacturers from Viatronix (Stony Brook, NY) to Vital Images (Plymouth, MN), and from GE Healthcare (Waukesha, WI) to Siemens Medical Solutions (Malvern, PA) and Philips Medical Systems (Bothell, WA), though it wasn't always so, he said. While a few manufacturers, such as Viatronix, have long had primary 3D reading capabilities, others have had to rush to upgrade their systems, according to Obregon. "They've scurried along very quickly to get their software up to speed," he said.
In remarks made to the ACR, Dr. Stephen Sener, president of American Cancer Society, focused on the potential benefits of an easier test for colorectal cancer screening.
"The ACRIN National CT Colonography Trial will evaluate the effectiveness of this noninvasive screening technology, and thus may help remove a barrier to getting more people 50 and older screened," Sener said. "If more people get screened, more lives will be saved from colorectal cancer."
By Eric Barnes
AuntMinnie.com staff writer
March 1, 2005
Related Reading
Missed lesions offer lessons in VC, January 17, 2005
Duke VC trial results disappointing, November 1, 2004
Gastroenterology warning: Prepare for VC or regret it, October 6, 2004
VC fares better as its own gold standard, September 8, 2004
Group credits 3-D reading for best-ever VC results, October 15, 2003
Multiobserver performance trial assesses utility of screening VC, November 27, 2002
Copyright © 2005 AuntMinnie.com