Pulmonary
Underdevelopment Syndromes:
View cases of pulmonary underdevelopment syndrome
Clinical:
Pulmonary agenesis refers to complete absence of a lung or lobe and its bronchi. Aplasia refers to absence of lung tissue, but rudimentary lobar bronchi are present. Hypoplasia refers to an underdeveloped lobe which contains both alveoli and bronchi.
Pulmonary
agenesis:
Approximately 1 in 15,000 children are born with congenital absence of one lung and the associated bronchus. Unilateral pulmonary agenesis most likely results from an in-utero insult during the 4th week of gestation [11]. Pulmonary agenesis occurs with equal frequency on the left and right side (other authors report it is more common [11] or twice as common on the left [3]). Right sided agenesis, however, is associated with a much worse prognosis due to greater anatomic distortion of the airway and great vessels, recurrent infections, and tracheobronchomalacia. Concomitant anomalies are frequently found (more than 50% of affected fetuses) including congenital heart disease, vertebral abnormalities, imperforate anus, renal agenesis, and tracheoesophageal fistula. [1,10]
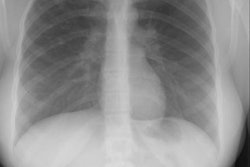
Pulmonary agenesis appears as an opaque hemithorax on the affected side [10]. There is mediastinal shift into the affected side, and compensatory hyperinflation of the remaining lung [10]. In right lung agenesis malposition of the aortic arch and descending aorta are caused by the extreme dextroposition resulting from the absence of the right lung. The distal trachea is commonly compressed and bowed posteriorly by the dextroposed crossing aortic arch. The left mainstem bronchus is also frequently compressed between the enlarged solitary left oulmonary artery anteriorly, and the malpositioned descending aorta posteriorly. The ipsilateral lung parenchyma, blood vessels, and airways are absent at CT [11].
Pulmonary
aplasia/hypoplasia:
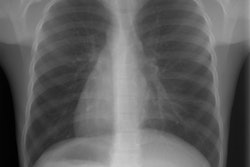
In pulmonary aplasia there is no lung present, however, there is a rudimentary bronchus that ends in a blind pouch [3]. In hypoplasia the alveoli and bronchi are present, but the involved lobe is small. The right upper and right middle lobes are most commonly involved [3]. The left lung is involved in about 25% of cases [3]. Although patients with pulmonary hypoplasia typically have minimal respiratory symptoms, the condition is commonly associated with other anomalies (seen in up to 60% of patients) including: Spinal anomalies (scoliosis, hemivertebrae), esophageal or diaphragmatic hernia, and pulmonary venolobar syndrome (or Scimitar syndrome). At CXR, the affected lung appears small and may be hyperlucent due to oligemia (alternatively, the contralateral lung may appear hyperlucent secondary to compensatory hyperexpansion) [11]. The ipsilateral pulmonary artery usually appears diminutive and ipsilateral medistinal shift is typically present [11].
Pulmonary Artery Interruption/Absence:
Interruption of the left or right pulmonary artery is an uncommon anomaly. In this congenital anomaly the proximal portion of the main pulmonary artery (arising from the primitive 6th aortic arch) fails to appear during embryologic development [9]. The term interruption is preferred to absence in view of the fact that it is usually only the proximal section of the vessel that is absent while the more peripheral intrapulmonary arterial network remains intact [4,7]. This is explained by the different embryologic origins of the proximal and distal pulmonary artery branches [7]. The intact intrapulmonary vessels receive oxygenated blood through systemic collaterals such as the bronchial, intercostal, or internal mammary arteries [8], or via a PDA [11].
In most cases the interrupted pulmonary artery lies on the side opposite the aortic arch- therefore, right sided interruption is more common [5,8]. Interruption of the left pulmonary artery is usually associated with a right sided aortic arch and other cardiovascular anomalies- most commonly tetrology of Fallot [4]. When the interruption is right sided, there may even be an anomalous artery arising from the ascending aorta [4]. Patients with right sided interruption have been grouped into three categories: 1- those having an isolated anomaly (most common); 2- those having an associated left-to-right shunt (usually a patent ductus arteriosus); and 3- those having associated pulmonary hypertension (PAH affects 19-25% of patients and is the most important prognostic indicator) [4,8]. Individuals in groups 2 and 3 are unlikely to survive beyond infancy, while those in group 1 often present as adults [4]. Recurrent pulmonary infection, hemorrhage, and mild dyspnea on exertion are the most common symptoms [8]. Hemoptysis occurs in about 10% of cases due to rupture of thin-walled, hypertrophied collateral vessels [4]. The hemoptysis is usually minor and self-limited [4].
Radiographic findings include an inapparent hilum on the affected side and an enlarged contralateral hilum. The ipsilateral lung is hypoplastic with volume loss, elevation of the hemidiaphragm, and mediastinal shift to the affected side [4]. Peripherally the ipsilateral lung is oligemic, but there is a reticular network of vascular markings which reflect the collateral arterial supply [7]. There is hyperinflation of the contralateral lung which herniates across midline [4,8]. Rib notching may be seen on the affected side if the collateral supply has developed from the intercostal arteries [7]. In addition to the other findings already described, CT will demonstrate a fine reticular increased attenuation within the periphery of the affected lung and serrated thickening of the pleura that represents trans-pleural collateral vessels [4].
Pulmonary
venolobar
syndrome
(PVS), Hypogenetic lung syndrome, or Scimitar
Syndrome:
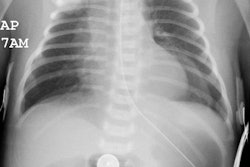
PVS is also known as Scimitar syndrome or hypogenetic lung syndrome. The disorder is most frequently characterized by unilateral pulmonary aplasia/ hypoplasia and ipsilateral total or partial anomalous pulmonary venous return (from the lower lobe) which drains into the inferior vena cava below the diaphragm or at the junction of the inferior vena cava at the right atrium (or less commonly to the hepatic, azygous, or portal vein, or into the right atrium). The draining vein produces a curvilinear vascular shadow which courses towards the hemidiaphragm and has the appearance of a Turkish sword (or scimitar). Since the anomalous vein drains blood from the lung into the inferior vena cava, a left-to-right shunt is established. Scimitar syndrome occurs almost exclusively on the right, with only one left-sided case report [3]. Other findings include: ipsilateral hypoplastic or absent central pulmonary artery (these patients will have a systemic arterial supply to peripheral pulmonary arteries of the affected lung from the descending thoracic or upper abdominal aorta), abnormal lobulation (bilateral left bronchial branching patterns), horseshoe lung, pulmonary sequestration, accessory hemidiaphragm, and cardiac dextropositioning due to the right lung hypoplasia.
An accessory hemidiaphragm is a thin membrane in the right hemithorax that is fused anteriorly with the hemidiaphragm and courses postero-superiorly to join the posterior chest wall. The membrane separates the right hemithorax into two parts- trapping all or part of the right middle or lower lobes beneath it. If the trapped lung is not aerated, the accessory hemidiaphragm and trapped lung appear as a solid mass along the right hemidiaphragm. If the trapped lung is aerated, an accessory fissure-like oblique line can be seen on the lateral chest radiograph. [3]
About 25% of affected patients have associated congenital heart disease [9]- most commonly a secundum type atrial septal defect.
Patients with PVS are usually asymptomatic, but may have recurrent pulmonary infections. Surgical correction is reserved for patients with a substantial left-to-right shunt.
In hypoplasia the heart and mediastinum are shifted towards the involved side (dextropositioning of the heart if right sided). There is compensatory overexpansion of the opposite lung. The thoracic cage is smaller on the affected side with approximation of the ribs, although this may not be evident at birth. The pulmonary vascularity on the involved side is usually diminished. A retrosternal opacity may be seen. This density has previously been erroneously ascribed to extrapleural areolar tissue anterior to the visceral pleura of the right lung. In actuality, this density is produced by the interface of the displaced mediastinum with the aerated, small right lung (Proto, ARRS 1996). In PVS a scimitar vein can be identified. On angiography there is an absent or hypoplastic ipsilateral pulmonary artery, and there may be a systemic arterial supply to the abnormal segment of lung.
Horseshoe
lung:
In horseshoe lung the right and left lungs are fused posteriorly by an ishmus of pulmonary tissue.
Horse shoe lung is an uncommon malformation that is typically associated with hypogenetic lung syndrome or lobar agenesis-aplasia complexes [7]. In horse-shoe lung a portion of the right lower lobe crosses the midline and is fused with the left lower lobe [7]. Typically, the fused segments are sheathed in a continuous layer of parietal pleura which forms a communication between the right and left pleural cavities [7]. Anatomically, this isthmus of pulmonary tissue is located posterior to the heart and anterior to the esophagus and descending aorta [7]. In most cases the arterial supply to the isthmus is from an anomalous branch of the right pulmonary artery [7]. The bronchi arise from the right bronchial tree [7]. Asymptomatic patients are managed conservatively [7]. The diagnosis can be confirmed at angiography which demonstrates a branch arising from the inferior aspect of the proximal right pulmonary artery crossing behind the heart to supply a part of the left lung [3].
REFERENCES:
(2) Radiology 1998; Cirillo RL. The scimitar sign. 206: 623-624 (No abstract available)
(3) Radiographics 1994; Woodring JH, et al. Congenital pulmonary venolobar syndrome revisited. 14: 349-369
(4) Radiology 2000; Davis SD. Proximal interruption of the right pulmonary artery. 217: 437-440
(5) Radiographics 2002; Zylak CF. Developmental lung anomalies in the adult: radiologic-pathologic correlation. 22: S25-S43
(6) Radiographics 2003; Konen E, et al. Congenital pulmonary venolobar syndrome: spectrum of helical CT findings with emphysis on computerized reformatting. 23: 1175-1184
(7) Radiol Clin N Am 2005; Paterson A. Imaging evaluation of congenital lung abnormalities in infants and children. 43: 303-323
(8) Radiographics 2006; Castaner E, et al. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. 26: 349-371
(9) Radiology 2008; Lee EY, et al. Multidetector CT evaluation of congenital lung abnormalities. 247: 632-648
(10) Radiographics 2010; Biyyam DR, et al. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and post natal radiologic-pathologic correlation. 30: 1721-1738
(11) Radiographics 2011; Dillman JR, et al. Expanding upon the unilateral hyperlucent hemithorax in children. 31: 723-741