Applying modified contrast-enhanced ultrasound (CEUS) criteria using perfluorobutane is effective in diagnosing liver cancer in high-risk patients, according to study published November 16 in the American Journal of Roentgenology.
A team led by Dr. Lingling Li from the Sun Yat-Sen University Cancer Center in Guangzhou found no significant differences between using this criteria and CT/MRI LI-RADS, suggesting that the CEUS method can be used as a first-line way to characterize or evaluate lesions.
"The modified CEUS criteria, compared with CT/MRI LI-RADS version 2018, showed no significant difference in terms of sensitivity, specificity, or accuracy in characterizing lesions as HCC in high-risk patients," Li and colleagues wrote.
Contrast-enhanced CT and MRI have served as the standard for noninvasive imaging tests in diagnosing hepatocellular carcinoma (HCC). However, CEUS has been explored in this area in recent years.
CT/MRI LI-RADS version 2018 (v2018) and CEUS LI-RADS version 2017 (v2017) both classify liver observations in high-risk patients according to the likelihood of HCC. The categories range from LR-1 (definitely benign) to LR-5 (definitely HCC).
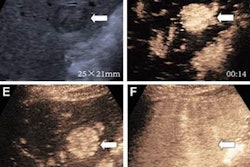
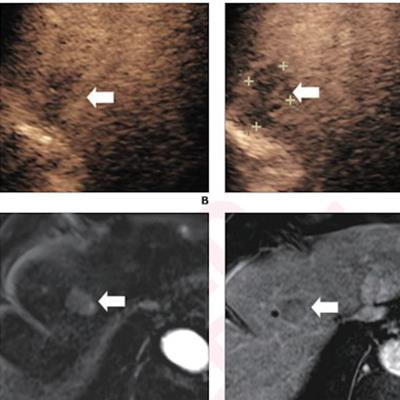
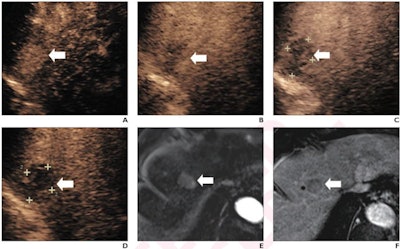 (A) Arterial-phase CEUS image shows a 25-mm segment-8 lesion with non-rim hyperenhancement (arrow). (B) CEUS image in portal-venous phase shows corresponding early washout (arrow). (C) CEUS image in late phase shows corresponding late and mild washout (arrow). (D) CEUS image in Kupffer phase shows marked Kupffer defect (arrow). The patient, a 71-year-old woman, also underwent evaluation by dynamic contrast-enhanced MRI using gadoxetate disodium. (E) Axial arterial-phase image shows 23-mm segment-8 lesion with non-rim arterial phase hyperenhancement (arrow). (F) Axial portal-venous phase image shows corresponding non-peripheral washout (arrow). This observation was classified as LR-M by modified CEUS criteria using perfluorobutane, and as LR-5 by CT/MRI LI-RADS v2018. The pathologic diagnosis based on surgical resection was intrahepatic cholangiocarcinoma. Images courtesy of ARRS.
(A) Arterial-phase CEUS image shows a 25-mm segment-8 lesion with non-rim hyperenhancement (arrow). (B) CEUS image in portal-venous phase shows corresponding early washout (arrow). (C) CEUS image in late phase shows corresponding late and mild washout (arrow). (D) CEUS image in Kupffer phase shows marked Kupffer defect (arrow). The patient, a 71-year-old woman, also underwent evaluation by dynamic contrast-enhanced MRI using gadoxetate disodium. (E) Axial arterial-phase image shows 23-mm segment-8 lesion with non-rim arterial phase hyperenhancement (arrow). (F) Axial portal-venous phase image shows corresponding non-peripheral washout (arrow). This observation was classified as LR-M by modified CEUS criteria using perfluorobutane, and as LR-5 by CT/MRI LI-RADS v2018. The pathologic diagnosis based on surgical resection was intrahepatic cholangiocarcinoma. Images courtesy of ARRS.The LR-5 category on CEUS shows "very high" specificity but has lower sensitivity than CT/MRI. But perfluorobutane microbubbles for CEUS have a particular phase called Kupffer that enhances liver parenchyma -- an effect not seen when CEUS is performed using conventional contrast agents, the group noted. They suggested that these Kupffer-phase findings could improve sensitivity in the LR-5 category.
Li and colleagues put this method to the test using modified LI-RADS against the performance of contrast-enhanced CT or MRI using LI-RADS v2018 for characterizing lesions as HCC in high-risk patients.
They included data from 171 patients. Of these, 114 had HCC, 43 had non-HCC malignancies, and 14 had benign lesions.
Researchers found no significant differences between the two methods.
| Comparison between CEUS, CT/MRI methods in characterizing HCC in high-risk liver patients (LR-5 category) | ||
| CT/MRI | CEUS | |
| Sensitivity | 89.5% | 92.1% |
| Specificity | 84.2% | 87.9% |
| Accuracy | 87.7% | 90.6% |
The investigators also reported that the modified CEUS criteria using perfluorobutane assessed one observation as LR-3 (benign lesion) and five as LR-5 (all HCC). These findings were originally categorized as LR-4 only by the CT/MRI method.
The researchers called for prospective multicenter studies with larger sample sizes to confirm their results but emphasized that the CEUS method has potential for first-line lesion characterization or for second-line evaluation after LR-4 or LR-M assessment by CT/MRI LI-RADS v2018.