CT developer Xoran Technologies said it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the company's Tron mobile fluoroscopy/CT scanner.
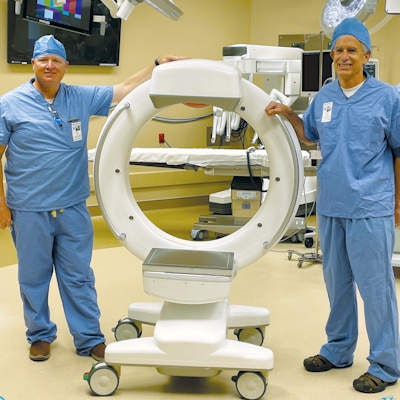
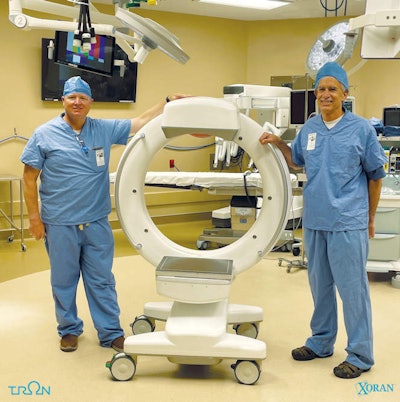 Xoran's Tron mobile CT/fluoroscopy system.
Xoran's Tron mobile CT/fluoroscopy system.Tron is a mobile unit that can be installed in vans or wheeled to patients and can produce scans in under a minute, the company said. Xoran believes the system will "democratize" access to medical imaging.
Xoran also recently began work on the second phase of a mobile lung research project with the goal of confirming the safety and utility of a future thoracic point-of-care CT system in support of an FDA submission. The company said that these efforts in lung CT are supported by a recent grant award from the U.S. National Heart, Lung, and Blood Institute (NHLBI) through the National Institutes of Health (NIH).