A large global study in stroke patients has shown how MRI biomarkers to estimate relative brain age can enhance surveillance and improve predictions on poststroke recovery, compared with simply using chronological brain age.
The research shows how radiomics-derived brain age can predict functional outcome after acute ischemic stroke. The results will be presented on May 5 in Lyon, France, at the European Stroke Organisation Conference (ESOC 2022). A preprint of the study (not peer-reviewed) is available.
The authors scrutinized the texture of brain T2 fluid-attenuated inversion recovery (FLAIR) images from 4,163 ischemic stroke patients across 17 sites in the U.S. and Europe to predict brain age. They then assessed the clinical determinants of accelerated brain ageing and, finally, its impact on poststroke functional outcomes.
 Dr. Martin Bretzner.
Dr. Martin Bretzner."Experienced clinicians usually assess the brain health of patients by looking at their brain parenchyma on T2-FLAIR MRI, then visually and subjectively quantifying the extent of atrophy and the cerebral burden of disease," lead investigator Dr. Martin Bretzner, stroke research fellow at Harvard Medical School in Boston, told AuntMinnieEurope.com.
We wanted to translate this process in a quantified framework, by analyzing T2-FLAIR MRI of stroke survivors with textural analysis, i.e., radiomics, and artificial intelligence," explained Bretzner, who is also an interventional neuroradiologist at Lille University Hospital, France. "One of our goals was also to provide a method that used clinical imaging and not research-grade imaging, the latter being used in most published research, which might fail to be applicable to MRI acquired during routine care."
Key findings
Stroke patients with older-appearing brains, characterized by a higher predicted brain age than chronological age, were more likely to suffer from hypertension, diabetes mellitus, or have a history of smoking or prior stroke. The older-appearing brains were also associated with cardiovascular risk factors, and they were also less likely to have favorable poststroke outcomes, according to Bretzner and colleagues.
The study, part-funded by the French Society of Radiology, found that relative brain age impacted stroke outcomes independently from chronological age and stroke severity. Having previously suffered from a stroke was the most influential clinical factor that impacted relative brain age, followed by diabetes.
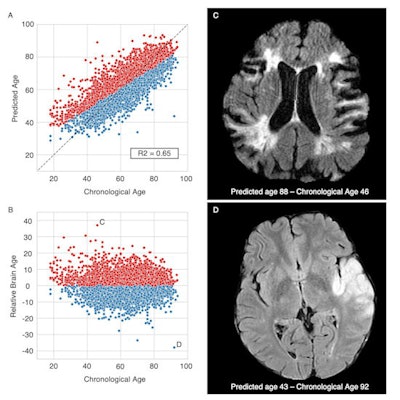 Brain age prediction performances and relative brain age. Scatter plots of the T2-FLAIR radiomics (A) predicted brain age and (B) relative brain age (RBA) per chronological age. Patients were colored in red if they had a positive RBA, so a brain that appeared "older" to their age-matched peers, or in blue if they had a negative RBA and a "younger" looking brain. (C) T2-FLAIR axial image of a patient with a positive RBA: predicted brain age = 88, chronological age = 46, RBA = 36.2; this patient's brain exhibits multiple cortical and subcortical sequelae, moderate to severe parenchymal atrophy with enlarged ventricles and sulci, and confluent white matter hyperintensities, which extents are unexpectedly large for a 46-year-old patient. (D) T2-FLAIR axial image of a patient with a negative RBA: predicted brain age = 43, chronological age = 92, RBA = -38.6; notwithstanding the left middle cerebral artery lesion, this patient's brain trophicity is maintained, the cortex and the deep gray nuclei are sharply defined, overall describing a healthy brain for this 92-year-old patient. Courtesy of Dr. Martin Bretzner and ESOC 2022.
Brain age prediction performances and relative brain age. Scatter plots of the T2-FLAIR radiomics (A) predicted brain age and (B) relative brain age (RBA) per chronological age. Patients were colored in red if they had a positive RBA, so a brain that appeared "older" to their age-matched peers, or in blue if they had a negative RBA and a "younger" looking brain. (C) T2-FLAIR axial image of a patient with a positive RBA: predicted brain age = 88, chronological age = 46, RBA = 36.2; this patient's brain exhibits multiple cortical and subcortical sequelae, moderate to severe parenchymal atrophy with enlarged ventricles and sulci, and confluent white matter hyperintensities, which extents are unexpectedly large for a 46-year-old patient. (D) T2-FLAIR axial image of a patient with a negative RBA: predicted brain age = 43, chronological age = 92, RBA = -38.6; notwithstanding the left middle cerebral artery lesion, this patient's brain trophicity is maintained, the cortex and the deep gray nuclei are sharply defined, overall describing a healthy brain for this 92-year-old patient. Courtesy of Dr. Martin Bretzner and ESOC 2022.Age is one of the most influential determinants of poststroke outcomes, but little is known about the impact of neuroimaging-derived biological "brain age," according to Bretzner, adding that the team's results show that quantifying relative brain age in stroke patients can be beneficial in assessing brain health globally and useful in predicting poststroke recovery.
The authors noted that chronological age, which simply measures the amount of time a person has lived, is less likely to capture accurately how well a patient has aged. By estimating the relative age of a patient's brain, this biomarker provides insight into the resilience of a brain to time, cardiovascular risk factors, and how well a patient will recover from stroke.
Such a biomarker would also be easy to discuss with clinicians and patients, as everyone instinctively understands the negative implications of an accelerated brain ageing process, they added.
In the pipeline
The authors noted that the study had several limitations, including a lack of T1-weighted images and the impossibility of exploring the relationship between T2-FLAIR radiomics-derived brain age biomarkers and more detailed outcome metrics such as cognitive or language outcomes. They pointed to how future studies could assess the relationship between radiomics brain age and cognitive reserve in stroke patients.
In the pipeline already, a prospective multicentric observational study of 8,000 ischemic and hemorrhagic stroke patients across 30 U.S. academic clinical sites will investigate the mechanisms of brain resilience and susceptibility to poststroke cognitive impairment and dementia.
Organized by the DISCOVERY (Determinants of Incident Stroke Cognitive Outcomes and Vascular Effects on RecoverY) team, the plan is to investigate the preexisting burden of cerebrovascular disease and neurodegenerative pathology, the impact of stroke lesions, and genetic variation on poststroke cognitive outcomes.
The principal investigators are Dr. Natalia Rost, chief of the stroke division at Massachusetts General Hospital (MGH) and professor of neurology at Harvard Medical School, and Dr. Steven Greenberg, PhD, director of the Hemorrhagic Stroke Research Program at MGH, and the DISCOVERY trial has already enrolled 600 patients.
How about clinical application?
Identifying potentially modifiable risk factors that impact brain health by using radiomics and relative brain age as a biomarker could lead to the development of stroke prevention interventions and aid recovery, noted Bretzner.
He hopes the work will raise awareness of the impact of brain health in stroke survivor prognosis.
"Our method could be used to further phenotype stroke patients upon admission," he said. "Brain age could be used as a brain health biomarker to personalize stroke care both at the acute stage and during follow-up."
Looking ahead, the team also hopes that brain age may be used as a biomarker of accelerated brain aging to identify patients at risk of stroke and enroll them into personalized primary prevention programs.
One in four stroke survivors will have another stroke and yet up to 80% might be prevented with the right treatments and lifestyle changes, according to the authors. In addition, the number of people living with stroke is estimated to rise by 27% between 2017 and 2047 in the European Union, mainly due to an increase in the number of people over 70.