Brain image analysis software developer ADM Diagnostics has received clearance from the U.S. Food and Drug Administration (FDA) for its CorInsights MRI quantitative image analysis software application.
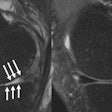
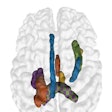
CorInsights MRI analyzes standard MRI exams to provide quantitative information about brain tissue volume for radiologists, neurologists, and researchers, according to the company. After the firm receives the image, the software measures the volume in certain regions of the brain and compares the results to a proprietary normative reference database that encompasses 600 scans. The findings are then translated into a set of images, graphs, and summaries. Results are sent back within the same day, ADM Diagnostics said.
The software was tested on more than 1,400 MRI exams from over 1,100 individuals, ADM Diagnostics said. The firm said it would begin accepting MRI scans for analysis by January 25, 2022.
In the future, the vendor hopes to develop the software to differentiate dementias, predict cognitive decline, and measure the effects of treatments.