There's more to F-18 FDG PET than meets the eye -- especially if radiomics features are added -- when imaging patients with diffuse large B-cell lymphoma (DLBCL), according to a study published August 18 in the European Journal of Nuclear Medicine and Molecular Imaging.
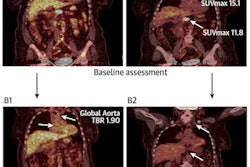
Dutch researchers pulled radiomics features -- standardized uptake values (SUV), dissemination features, and sphericity, or the measure of the roundness of tumors, for instance -- in patients with diffuse large B-cell lymphoma who had F-18 FDG PET scans. The group found the additional features significantly improved their ability to identify patients at high risk of relapse.
In the short term, the finding could improve clinical trial designs. In the long-term, it could help identify patients who need new treatments the most, the researchers stated.
"Prediction models using baseline radiomics combined with currently used clinical predictors identify patients at risk of relapse at baseline and significantly improved model performance," wrote Jakoba Eertink of Amsterdam University Medical Center.
Diffuse large B-cell lymphoma is the most common subtype of aggressive non-Hodgkin lymphoma in adults. Studies suggest up to one-third of patients fail to achieve complete remission during first-line treatment or experience relapse.
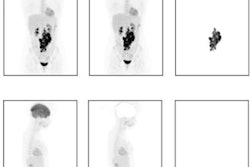
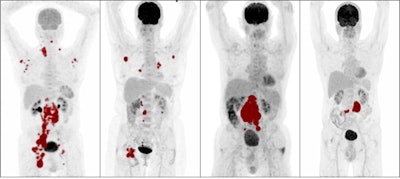 Maximum intensity projections of patients with high metabolic tumor volume (MTV), low MTV, high dissemination, and low dissemination. Tumor delineations are indicated in red. From left: high MTV and high dissemination, low MTV and high dissemination, high MTV and low dissemination, and low MTV and low dissemination. Image courtesy of the European Journal of Nuclear Medicine and Molecular Imaging.
Maximum intensity projections of patients with high metabolic tumor volume (MTV), low MTV, high dissemination, and low dissemination. Tumor delineations are indicated in red. From left: high MTV and high dissemination, low MTV and high dissemination, high MTV and low dissemination, and low MTV and low dissemination. Image courtesy of the European Journal of Nuclear Medicine and Molecular Imaging.Current clinical scoring systems, such as the international prognostic index (IPI), fail to identify high-risk patients who need novel treatment approaches the most, according to the authors. In this study, the researchers aimed to evaluate the added value of baseline radiomics features in DLBCL patients compared with IPI scores.
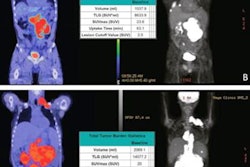
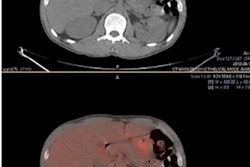
The researchers analyzed FDG PET/CT data from 317 newly diagnosed DLBCL patients who received baseline imaging at 58 hospitals as part of a phase III trial of a new monoclonal antibody treatment in 2020. Using a software program, they extracted 490 additional radiomics features from the PET scans, including simple features such as lesion SUV levels and the shape of tumors.
The researchers then developed six models based on combinations of IPI components and additional radiomics features, models including metabolic tumor volume (MTV), SUV levels, dissemination features, and sphericity. They then compared the models for predicting which DLBCL patients were likely to relapse after two years of first-line treatment.
They found the optimal radiomics model comprised the natural logarithms of MTV and of SUVpeak and the maximal distance between the largest lesion and any other lesion (Dmaxbulk; AUC, 0.76).
Combining radiomics and clinical features showed that a combination of tumor- (MTV, SUVpeak and Dmaxbulk) and patient-related parameters (World Health Organization performance status and age > 60 years) performed best (AUC, 0.79).
Adding radiomics features to clinical predictors increased positive predictive value, with more accurate selection of high-risk patients compared with the IPI model (44% vs. 28%).
"Results from study indicate that baseline radiomics features are predictive of outcome and have added value compared to currently used clinical parameters," the researchers wrote.
Radiomics is an emerging field, the authors noted. Yet baseline F-18 FDG PET/CT is already part of clinical practice and radiomics features can be calculated at no additional costs. In practice, feature extraction means simply pressing the "run" button and waiting for the computation to be finished.
With software becoming available that easily and reliably calculates radiomics features, adding radiomics features to clinical scoring systems should seriously be considered, the authors suggested.
"These results will be validated in a large cohort of DLBCL patients treated in different clinical trials," namely the PETRA project, which is investigating the role of interim FDG-PET in malignant lymphoma, the researchers concluded.