A CT-based radiomics model helped clinicians predict response to neoadjuvant chemotherapy in patients with advanced gastric cancer, according to a study published August 19 in JAMA Network Open.
A team of researchers led by Dr. Wei Wang, PhD, of Sun Yat-sen University Cancer Center in Guangzhou, China, found 20 CT radiomic features that indicate whether pretreatment chemotherapy in gastric cancer patients will be successful -- findings that could improve treatment planning and care.
"Given that no patients respond equally to neoadjuvant chemotherapy, the radiomics signature proposed in this study could guide clinicians in selecting appropriate patients for neoadjuvant chemotherapy," the group wrote.
Gastric cancer tends to have a high mortality rate. It is often treated with chemotherapy before gastrectomy, as presurgical chemotherapy has been shown to reduce tumor size and improve outcomes. But not all patients respond to pretreatment in the same way, and for the sake of effective patient care, it's important to assess who will respond to pretreatment and who will not.
In previous research, Wang's group developed and tested a radiomics nomogram -- a model that incorporates biologic and clinical characteristics to predict medical outcomes -- to forecast preoperative lymph node metastasis in people with colorectal cancer. The nomogram extracts features from CT images such as shape, texture, and gray levels and integrates them with other elements like carcinoembryonic antigen (CEA) levels and whether cancer has spread to lymph nodes.
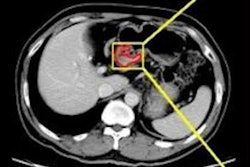
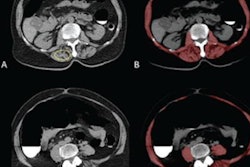
The group conducted a study that included 323 patients with advanced gastric cancer (local, not metastasized) who were treated between January 2010 and July 2017 at three hospitals. Patients underwent CT before receiving a course of neoadjuvant chemotherapy, and Wang's team examined the findings for diagnostic features that might predict study participants' chemotherapy response.
The researchers also assessed information from patients' clinicopathological data and pathology reports. Of the study participants, 250 were in a training group (77%) for the radiomics model and 73 were in a validation group (22.6%). The team measured the reliability of clinicopathological and radiomic features for predicting patient response to pretreatment chemotherapy using the area under the receiver operating characteristic curve (AUC).
The number of patients who responded to pretreatment chemotherapy was 122 (48.8%) in the training group and 40 (54.8%) in the validation group, while the number of patients who did not respond to pretreatment chemotherapy was 128 (51.2%) in the training group and 33 (45.2%) in the validation group.
The investigators did not find any clinicopathological variables that predicted treatment response, but they did find 20 CT radiomic features out of a total of 7,477 that showed positive association with patients' response to neoadjuvant chemotherapy. In the training group, the radiomics signature showed an AUC of 0.736, while in the validation group, it showed an AUC of 0.679.
"Radiomics was able to bridge radiology and histopathology by using postoperative pathological findings as a reference standard to identify potential responders for neoadjuvant chemotherapy," Wang and colleagues wrote.
The study findings could not only translate into better care for advanced gastric cancer patients but also offer radiologists a way to support their oncology peers, according to the authors.
"[This radiomics signature] could be used to offer evidence-based guidance, rather than solely relying on clinical judgment, for multidisciplinary consultation between radiologists and oncologists to predict pretreatment response and offer justifiable individualized therapeutic options to these patients," they concluded.