Lantheus Medical Imaging has announced that oncology provider GenesisCare will be the first to administer commercially available doses of its Pylarify PET radiopharmaceutical, which was approved last month.
Pylarify (F-18 DCFPyL) was designed to target prostate-specific membrane antigen (PSMA). The agent is labeled with F-18 and homes in on PSMA, a protein that is overexpressed on the surface of more than 90% of both primary and metastatic prostate cancer cells.
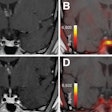
PET imaging detects accumulations of Pylarify at sites of prostate cancer, making it useful for detecting metastasis from primary cancer or recurrence of disease in men who have already been treated, according to the company.
GenesisCare reports its clinical teams treat more than 400,000 people at more than 440 locations worldwide each year.