The U.S. Food and Drug Administration (FDA) has cleared medical equipment manufacturer Aspenstate's portable x-ray device, AiRTouch.
The system is an effective tool for diagnosing COVID-19, Aspenstate said. The handheld, 5.5-lb device transmits x-ray images to a facility's PACS via a built-in workstation. It can capture up to 300 exposures in a single battery charge, and its battery charges within two hours, according to the firm.
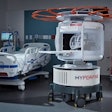
 AiRTouch includes a built-in touchscreen workstation. Image courtesy of Aspenstate.
AiRTouch includes a built-in touchscreen workstation. Image courtesy of Aspenstate.AiRTouch was developed by Aspenstate's parent company, Livermoretech Korea, and has been used at drive-through COVID-19 screening centers in South Korea, Aspenstate said.