Carestream Health has received 510(k) clearance from the U.S. Food and Drug Administration on its dual-energy imaging technology for digital radiography (DR) and its Focus 35C detector with Image Suite software.
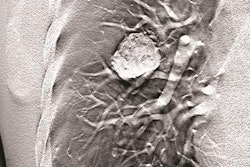
The dual-energy technology is approved for Carestream's DRX-Evolution Plus DR system and features the Eclipse image processing engine and two filter materials designed to automatically switch between the high- and low-energy exposures. By doing so, it produces a soft tissue-only image with the bone structures removed and a corresponding bone-only image, which enhances both x-ray spectrums to reach dose efficiency.
Carestream's new Focus 35C detector with Image Suite software targets smaller imaging facilities and specialty practices with a retrofit option to convert analog x-ray rooms to wireless digital radiography for enhanced image processing and broader functionality. The detector and software also combine to provide a mini-PACS.
Carestream expects the Focus 35C detector to be commercially available by the end of this year, while the dual-energy technology's release is planned for early 2020.