Medical artificial intelligence (AI) software developer Qlarity Imaging has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its QuantX MRI guidance breast biopsy software plugin.
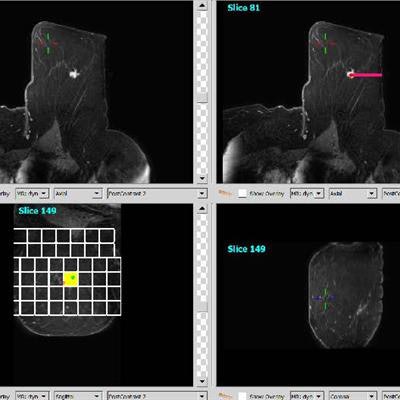
The MRI guidance breast biopsy software plugin is the latest addition to Qlarity's QuantX breast imaging computer-aided diagnosis platform. The plugin allows radiologists to plan MRI-guided breast interventions such as breast biopsy. It uses information from MR images to calculate the precise location and depth of a particular lesion and then recommends the ideal location and depth for inserting a biopsy needle.
Qlarity Imaging is a subsidiary of Paragon Biosciences.
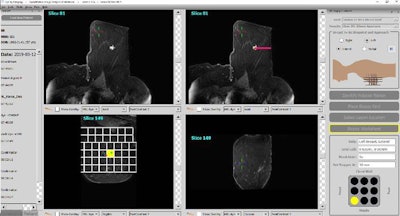 QuantX MRI guidance breast biopsy software plugin. Image courtesy of Qlarity.
QuantX MRI guidance breast biopsy software plugin. Image courtesy of Qlarity.