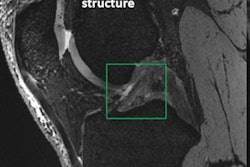
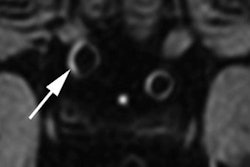
The increasing utilization of 7-tesla MRI in the clinical setting has prompted a group of safety experts to call for the creation of specific standardized tests and recommendations for ultrahigh-field MRI scanners to prevent harmful events for patients and staff. The initiative is detailed in an article in the September issue of Radiology.
Among their concerns is how the strength of the 7-tesla magnet could affect the function of patients' metallic implants and the "unpredictable" radiofrequency (RF)-induced heating of tissue near devices. Another issue is that patients have reported vision problems, dizziness, nausea, and vertigo after undergoing a 7-tesla MRI scan.
"It is hoped that testing standards and the number of implants evaluated according to [those standards] will be updated to enable a greater number of patients with biomedical devices to have access to 7-tesla MRI," wrote the advocates, led by Michael N. Hoff, PhD, from the University of Washington in Seattle (Radiology, September 2019, Vol. 292:3, pp. 509-518). "Regardless, it is the responsibility of the institution that installs a 7-tesla clinical MRI scanner to ensure patient safety both before and during the imaging process, and ideally to report adverse effects to add to the growing body of 7-tesla safety knowledge."
Soon after the U.S. Food and Drug Administration (FDA) cleared the first 7-tesla MRI scanner for clinical imaging, several institutions expanded their use of the modality to evaluate patients with neurological disorders, such as multiple sclerosis and epilepsy, and better diagnose musculoskeletal injuries. At the same time, clinicians became acutely aware of patients with implants and other accessories that might prevent the completion of a 7-tesla scan.
In this paper, Hoff and colleagues cited metallic implants as one primary complication with 7-tesla MRI, given their "high conductivity and low resistance to electric currents." Previous research has shown that 7-tesla scanning usually leads to minimal heating of implants, but surrounding tissue may experience increased heated due to relatively high electrical resistance. Implants and devices that are associated with nominal heating at 1.5- and 3-tesla "may trigger unsafe heating at 7-tesla," they noted.
Given that only 300 or so metallic implants and RF transmit coils have been tested for safety at 7-tesla MRI, compared with more than 6,000 metallic devices that have undergone evaluation at 1.5- and 3-tesla imaging, it is "apparent that 7-tesla-specific guidelines and further testing are needed to ensure safe 7-tesla imaging in the future," the authors wrote.
Regarding patient comfort, Hoff and colleagues suggested that technologists should consider increasing the amount of time patients spend in the 7-tesla static magnetic field as they enter and exit the scanner to help alleviate any physical issues. In addition, earplugs and headphones could help reduce acoustic noise and related inner-ear and vision abnormalities. Scanning sequences using acoustic noise reduction techniques are currently offered by most vendors, they added, although not all pulse sequences currently are available with such options.
"After considering the safety issues that are unique to MRI at 7 tesla relative to lower magnetic field strengths, it is apparent that safety guidelines specific to this high-field strength should be established," the authors concluded. "Many of the safety considerations discussed herein also apply to magnetic fields higher than 7 tesla, although the recent adoption of clinical 7-tesla MRI necessitates the standardization of 7-tesla-specific safety recommendations."