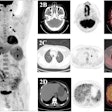
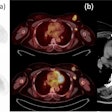
Imaging software developer Mirada Medical has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Simplicit90Y dosimetry software.
In collaboration with BTG Interventional Oncology, Mirada developed Simplicit90Y to improve the standardization and reproducibility of dosimetry planning and post-treatment validation for workflow in transarterial radioembolization (TARE) procedures, which use yttrium-90 (Y-90) to treat cancer.
The software offers digital processing, image manipulation, quantification analysis, and various other features to assist in the review and reporting of medical images. These features may facilitate personalized treatment for patients with liver cancer who need to undergo a TARE procedure, according to the companies.
Simplicit90Y previously received the CE Mark for clinical use in Europe as well as approval for use in Canada.